Chemical bonds is one of the topics assigned to Grade 9 Science by the Philippine Department of Education (DepEd).
As to the title, the word "manuscript" is used instead of "draft" just for convenience . Strictly speaking, this document ceased to be a manuscript because of its publication and be described appropriately as draft.
TOPIC OUTLINE
LESSON 1 REVIEW ON ATOMIC STRUCTURE AND PERIODIC TABLE
LESSON 2 PERIODIC TRENDS
LESSON 3 ELECTRON CONFIGURATIONS
LESSON 4 IONIC BONDS
LESSON 5 COVALENT BONDS
LESSON 6 LEWIS FORMULAS
LESSON 7 VSEPR AND MOLECULAR SHAPES
LESSON 8 METALLIC BONDS
LESSON 9 INTERPARTICLES ATTRACTIVE FORCES
LESSON 1
REVIEW ON ATOMIC STRUCTURE AND THE PERIODIC TABLE

Required Material: Periodic Table
Suggested Time Frame
5 contact hours
N.B. In this part of the module, the teacher is required to discuss and clarify the concepts of atomic structure and the use of the periodic table to the students..
Text
Hello! You are at ground zero. Here, the module with help you understand the world of chemical bonding. Bonding, can be understood, as a sort of doing something together ( enjoying together) with family members and friends. Merriam Webster defines bonding as "the formation of relationship" also "the attaching of a material." In short, bonding is a form of attachment. One being emotionally attached with friends or with family members and, as a result, one feels relax and happy. Similarly, atoms (though they do not have emotions) in order to become stable, they bond with each other. Atoms do their bondings by either sharing its outermost electrons (covalent bonding) or by making themselves charged ( + or -) through the transfer of their outermost electrons (ionic bonding). Ok ok ok, but before you feel sleepy and to have a better understanding of chemical bonding, let us look at the atom first. What is an atom? Has anyone seen an atom? Interesting isn’t it?
1.1 A Review about the Atom
In the 1800’s John Dalton proposed that an atom is spherical in shape and massive. We can compare his proposed atom as that of a massive billiard ball. It was only in the 1980’s with the use of an electron microscope called scanning tunneling electron microscope (STM) that scientists were able to get a picture of an atom. Indeed, Dalton was right. Atom has spherical shape. Other experiments, however, such as that of Rutherford’s experiment proved that an atom is NOT massive as described by Dalton.
Atom is the smallest particle of an element. This means an element has atoms of the same kind, that is, of the same atomic number.. For example, a phosphorus element has phosphorus atoms ( atoms of the same atomic number, but with different atomic mass-called Isotopes). We can say that if you subdivide an element (e.g. a pure gold bar) into its smallest portion, you will end up with a particle that is a representative of that element, the atom. The atom is so tiny that it cannot be seen by our naked eyes so that an individual atom has no color, only visible objects (those that can be seen) have colors. However, many atoms bonded together form bigger particles big enough to be seen with colors such as a silver element with brilliant gray color, gold element that has a metallic yellow color, etc. Also, when atoms are excited and go back to their original state (ground state) , they can emit characteristic colors such as in flame test ( sodium gives a yellow flame, copper bluish green, calcium brick red, etc.)
Interesting to note, the first (on record) to think of dividing an object into its smallest portion is a philosopher named Leucippus. Later, Democritus called the smallest particle of an object “atomos.”
1.11 Nucleus, Energy Shells, and Subatomic particles
So, the atom is spherical in shape. This sphere has two parts: the nucleus and the energy shells where the subatomic particles reside or can possibly be found (Table 1). Electrons reside in the energy shells/levels while protons and neutrons in the nucleus. What defines the shape of the atom is where the subatomic particles can possibly be found. The shape of the atom is the space where you can possibly find the subatomic particles. Thus, it follows that “No particles, No shape, No atom.”
Table 1 The Principal Subatomic Particles
|
Particles |
charge |
symbol |
Location |
Mass (amu) |
|
Electron |
(-) negative |
e- |
energy shell (outside the nucleus) |
0.0005486 |
|
Proton |
(+) positive
|
p+ |
Inside the nucleus |
1.0073 |
|
Neutron |
No charge
|
no |
Inside the nucleus |
1.0087 |
There are other particles in an atom, but we will only take up the three principal subatomic particles of an atom, the proton, neutron, and electron. This is because knowing them will lead us to a better understanding of how atoms join together to form compounds.
1.12 Where exactly is an electron? Heisenberg Uncertainty Principle
No one can exactly locate an electron. We can only talk of the possibility ( probability) that the electron is in a particular area or region in space. Thus, we say, an electrons can be found somewhere outside the nucleus (energy shells). We cannot say, an electron is in that particular point outside the nucleus. In short we can only tell the possibility or probability of finding an electron outside the nucleus. Being not sure where exactly is the electron is called the Heisenberg Uncertainty Principle.
Heisenberg Uncertainty Principle simply states that no one can exactly measure the position and momentum (quantity of motion) of an electron at the same time. Thus, we can only say, electrons are somewhere outside the nucleus.
Neils Bohr model of an atom, the micro-planetary system, no longer holds true. It is now replaced by the electron cloud model, owing to Heisenberg Uncertainty Principle and Schrodinger wave equation.
1.13 Highest probability of finding an electron.
Let us liken the location of an electron to where you are (let just say, your name is Robert). If right now at home, somebody asks your mother where you are, your mother will probably say, “Robert is in school” without exactly (precisely) knowing where you are inside the school. If the guard at the gate of the school will be asked, where Robert is, the guard will probably say, “Robert is in that building” without exactly knowing where you are exactly inside the building. Now, if somebody asks a student in that building where Robert is, the student will probably say, “ Robert is in that classroom” without exactly knowing where you are seated inside the classroom.
So, where can we find Robert with the highest probability?
A. in the school B. in the building C. in the classroom
1.14 Spaces in the Atom: Energy level, Sublevel, Orbital
In an atom, the spaces where electrons can possibly be found are given names. They are the energy level (school), sublevel (building), and orbital (classroom).
So, at highest probability, where can we find an electron?
A. Energy level B. Sublevel C. Orbital
.Practice Task 1-A Understanding the Atom
Name:_________________________________ Score:______________
Class Schedule:_________________________ Date:_______________
Understanding the Atom
A. Fill in the blanks in the paragraph below with the correct answer. Choices are given inside the parentheses. Write your answers in the blanks provided.
An atom is ___________(spherical/square) in shape. It has three principal subatomic particles namely, the electrons, protons, and neutrons. A proton has a ___________ (positive/negative/no) charge, an electron has _________(positive/negative/no) charge, and neutron has a ___________(positive/negative/no) charge. The lightest among the subatomic particles is the __________ (electron/proton/neutron) while the heaviest is the __________ (electron/proton/neutron). Electrons can be found ___________ (inside/outside) the nucleus while the protons and neutrons can be found ____________(inside/outside) the nucleus. The nucleus is the ___________(heavier/lighter) part of the atom. The nuclear charge (charge of nucleus) is __________ (positive/negative).
We can only talk of the probability of finding an electron as inferred by _____________________ principle. Thus, electrons can be found with highest probability in the ______________(energy shell/sublevel/orbital).
Practice Task1-B Understanding the Atom
Name:_________________________________ Score:______________
Class Schedule:_________________________ Date:_______________
B. Based on your understanding of the shape of an atom, draw or sketch an atom showing the nucleus, protons, electrons, and neutrons. ( First, recall the following; the shape of atom according to Dalton, the location of the nucleus and energy shells in an atom, and the location of the protons, neutrons and electrons. Now, start drawing on the space below. Do not be afraid that your drawing will not look good because “if it is yours, it must be good!!!”
Practice Task 1-C Understanding the Atom
Name:_________________________________ Score:______________
Class Schedule:_________________________ Date:_______________
C. Given pictures (from the teacher) of Bohr Model and Electron Cloud Model of atom, describe how the two models differ from each other and give reasons for the differences.
1.20 Review about the Periodic Table
Note: Be ready with your periodic table
1.21 Periods and Groups
Like any ordinary tables, the Periodic Table has rows (horizontal/from left to right) and columns (vertical/from top to bottom). However, in the Periodic Table the rows are called PERIODS, while the columns are called GROUPS.
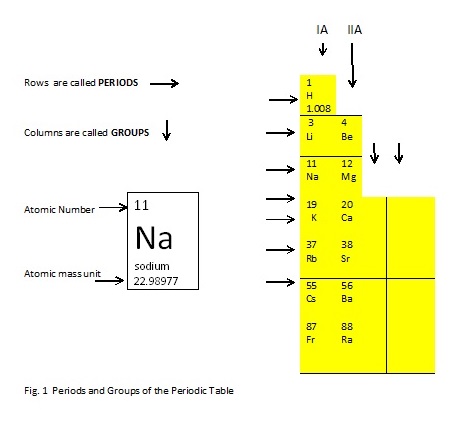
Period number = number of energy shells
Group number = number of valence electrons (electrons at the outermost energy shells)
Note: The group number is directly equal to its valence electrons. This is true only for representative elements. Have you forgotten where in the periodic table, the representative elements are? Please feel free to ask your teacher politely and then thank him/her.
The period number is the number of energy shells of an atom, the group number is the number of electrons at the outermost (valence) energy shell or the number of the outermost electrons called valence electrons. It follows that by looking at the location of an element in the periodic table, one will know its period number and the group number
Refer to Fig. 1 above, complete the table below by writing the period number, group number, number of energy shells, and number of valence electrons.
Sample Task 1
|
Atom or Element |
Row Location /Column label |
Period No. |
Group No. |
Number of Energy Shells |
Number of Valence Electrons |
|
Sodium (Na) |
3rd row/ IA |
3 |
IA |
3 |
1e- |
|
Barium (Ba) |
6th row/ IIA |
6 |
IIA |
6 |
2e- |
|
Magnesium (Mg) |
|
|
|
|
|
|
Potassium (K) |
|
|
|
|
|
1.22 The Periodic Law and Periodic Table
Periodic table is a list of elements arranged according to atomic number, grouping those with similar properties ( physical and chemical properties) in vertical columns. Periodic law says that there is a periodic recurrence of similar properties when the elements are arranged according to increasing atomic number. This law became the basis of the modern periodic table. Thanks to Henry G.H. Moseley in his study on x-ray spectra of elements leading to this periodic law.
Atomic Number = Number of protons
A Periodic Table is a list of elements arranged according to similar properties (in vertical columns) and thus, arranged in increasing atomic number.
Atomic number is the number of the protons in the nucleus of an atom of a particular element. So that sodium (Na) whose atomic number is 11, has 11 protons; and barium (Ba) with atomic number of 56 has 56 protons.
Atomic Mass unit (amu) and Mass Number
Atomic mass unit is the mass of an atom relative to carbon -12, that is, it is the mass of an atom as compared to carbon -12. The relative atomic mass unit is determined using an instrument called mass spectrogram. Atomic mass unit is usually in decimal number.
Mass number is the total number of protons and neutrons in the nucleus of an atom. The number of protons and neutrons are numbers from counting pieces of protons and pieces of neutrons,, it is always a whole number. The mass number is in whole number, being a total number of protons and neutrons.
Since the mass of an atom is greatly contributed by the masses of protons and neutrons in the nucleus, it follows that the atomic mass unit is the mass of the nucleus. So that, to determine the mass number, you just have to round off the atomic mass unit to its nearest ones (to its whole number).
Sample Task 2
Following the first two examples in the table, complete the table below by writing the atomic mass unit and mass number of atoms of Magnesium (Mg) and Potassium (K). Refer to Fig. 1 above
|
Atom in the Element |
Atomic Mass Unit |
Mass Number |
|
Sodium (Na) |
22.9898 |
23 |
|
Barium (Ba) |
137.327 |
137 |
|
Magnesium (Mg) |
|
|
|
Potassium (K) |
|
|
Notice that the mass number is the total number of protons and neutrons in the nucleus, It follows that the number of neutrons is the difference between the mass number and the atomic number ( Number of neutrons = Mass number – Atomic number).
Sample Task 3
Following the examples in the Table, complete the table by writing the number of neutrons, mass number and atomic number(Refer to Fig. 1 above)
|
Atom in the Element |
Atomic Number |
Mass Number |
Number of Protons |
Number of neutrons |
|
Sodium (Na) |
11 |
23
|
11 |
23-11 = 12 |
|
Barium (Ba) |
56 |
137
|
|
137-56 = 81 |
|
Magnesium (Mg) |
|
|
|
|
|
Potassium (K) |
|
|
|
|
1.23 Number of electrons in an atom
The number of electrons depends on whether the atom is neutral or charged(+/-). Charged atoms are called ions. If the atom is neutral, the number of protons is equal to the number of electrons. If the ion is positively charged, the number of electrons is the atomic number minus the number of positive charges. If the ion is negatively charged, the number of electrons is the atomic number plus the number of negative charges. Take note that the atomic number and the number of protons do not change even when the atom is charged.
Example: Number of Electrons, atomic number, and charges. (Refer to a complete periodic table)
|
Atom in the Element |
Atomic Number |
Charge |
Number of Electrons |
|
Sodium (Na) |
11 |
0 |
11 |
|
Sodium ion (Na1+) |
11 |
1+ |
11-1 = 10 |
|
Barium (Ba) |
56 |
0 |
56 |
|
Barium ion ( Ba2+) |
56 |
2+ |
56 – 2 = 54 |
|
Sulfur (S) |
16 |
0 |
16 |
|
Sulfide (S2-) |
16 |
2- |
16 + 2 = 18 |
1.24 Division in the Periodic Table
Elements in the periodic table are mainly divided into three: The representative elements, the transition elements, and the inner transition elements.
Groups are also called families. The following are the representative elements or the Group A elements:
1. Group IA is the Alkali metals
2. Group IIA is the Alkaline Earth metals
3. Group IIIA is the Boron family
4. Group IV A is the Carbon family
5. Group V is the Nitrogen family
6 Group VI is the Oxygen family
7. Group VII is the Halogen family
8. Group VIII is the noble gas family

Test Yourself
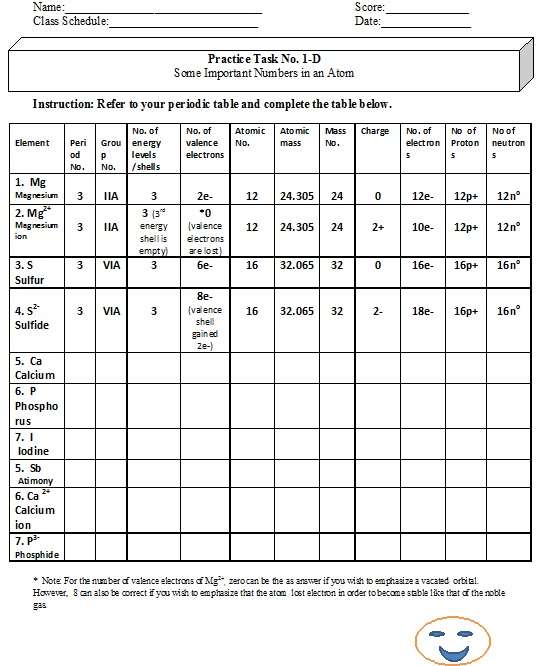
* Note: For the number of valence electrons of Mg2+, zero can be the as answer if you wish to emphasize a vacated orbital. However, 8 can also be correct if you wish to emphasize that the atom lost electron in order to become stable like that of the noble gas. However, I prefer "zero" as answer to indicate lost of valence electrons as it is a cation.
![]()
![]()
![]() PERFECT!!! CONGRATULATIONS! YOU MADE IT.
PERFECT!!! CONGRATULATIONS! YOU MADE IT.
References:
Bonding (2015) Meriam Webster retrieved from http://www.merriam-webster.com/dictionary/bonding on July 7, 2015.
Brown and Holme (2012) Chemistry for Engineering Students, 2nd ed. Singapore: Cengage Learning Asia, Pte Ltd.
Brown, Lemay, and Burstein (2007), Chemistry the Central Science, 10th ed. Upper Saddle River, New Jersey: Prentice Hall, Inc.
McMurry, J and Castellion, M.E. (1996). Fundamentals of General, Organic, and Biological Chemistry. Upper Saddle River, New Jersey: Prentice Hall, Inc.
Petrucci, Hardwood, and Herring (2004). General Chemistry Principles and Modern Applications. Upper Saddle River, New Jersey: Prentice Hall, Inc.
Timberlake, K. (2010). General, Organic and Biological Chemistry: Structures of Life. Singapore: Pearson Education, Inc. publishing as Prentice Hall.
Zumdahl, S. (2000). Introductory Chemistry A Foundation. USA: Houghton Mifflin Company.




Comments