Marta Santos took the plastic cage from my hands and smacked it with the palm of her hand, causing a few lifeless bodies to fall off the walls of the cube and collect on the floor. It was like kicking a vending machine so that the Snickers bar would drop into the compartment below. I took the cage back and, following suit, smacked it a few times, then watched the living fruit flies, or Drosophila melanogaster, whiz frantically around the confines of their plastic home, disturbed. I used a paintbrush to collect the dead and place them into a small plastic cap on the floor of the cage. They had, by experimental design, starved to death.
Marta put a plate of new food—a mixture of banana, yeast, and corn syrup—into the cage. Now we had to identify how many males and females had died. Sliding the bodies under the microscope, I squinted through the lens and prodded them until I counted six females and two males, distinguishable by the females’ elongated bellies and the males’ small, rounded bellies.
This was a test of starvation resistance. By breeding the surviving flies—the ones that survive despite a lack of food—scientists like Santos and others at UCI’s famed Rose Lab have created a miniature display of natural selection. Santos is a Junior Researcher from Portugal. Since moving to Irvine in 2010, she has been working on her research in order to get her PhD in Portugal. She supervises 58 students doing tests similar to the one I just performed: they count fruit flies, prepare food, clean the lab, and log data for twelve hours a week, including weekends.
The research is the product of over more than 30 years of immortality research using fruit flies, an approach started in the 1930s but brought into today’s scientific world by Santos’ supervisor, Dr. Michael Rose. Over the years, Rose and his lab have bred fruit flies to live four times the life span of an average fruit fly. Reasoning from those studies, Rose has proposed that, because the life spans of fruit flies have the genetic capability to be extensively prolonged, human life can be manipulated in the same way.
In other words, biological immortality is entirely possible.

Watercolor illustration of Drosophila by Edith M. Wallace, Thomas Hunt Morgan’s illustrator. This image was published in C.B. Bridges and T.H. Morgan, Contributions to the Genetics of Drosophila melanogaster (Washington, DC: Carnegie Institution; 1919), CIW publication
Rose did not set out to be an evolutionary biologist on the hunt for some elixir of life. A native of Canada, his career began as a PhD student at the University of Sussex, where he studied aging under evolutionary biologist Brian Charlesworth. Charlesworth had made his name in the field of ecology and evolution and he was the first to argue to Rose that evolutionary biology could solve the problem of aging. Charlesworth’s key insight was based on the fall of the force of natural selection that takes place in late life. In his own book, The Long Tomorrow, he explained the phenomena.
Natural selection, he writes, is like a bouncer at the evolutionary nightclub used by teenagers. It throws out the badly behaved; it’s strong enough to throw out bad mutations. At late ages, however, natural selection is weak. It stops caring, letting harmful mutations affect the elderly: “Natural selection becomes more like a nursing home orderly waiting indifferently for his elderly charges to die.” Natural selection has become “an underachiever” at this point. It’s stopped caring about the elderly.
Charlesworth saw that this evolutionary pattern meant that more trouble-causing genes would be preserved in the later years, but fewer bad genes would affect the young. There would be more genetic variation (resulting in adverse effects) later in life in groups of aging animals. Youth, he wrote, would be “monotonously perfect because of fine-tuned genetics”.
This prediction of an increase in genetic variation with age was Rose’s first test of Charlesworth’s hypothesis. He began the experiment in the spring of 1977, using a deceptively simple protocol: He would study the number of eggs that females laid in a day to study fecundity, their measure of biological health. Rose and Charlesworth hypothesized that they would find little genetic variation that affects egg laying in younger flies because natural selection would eliminate mutations that impair egg laying in flies of a young age. Natural selection would weaken for older flies and cause more bad genes in their population. Rose finished the study in the summer of 1978. He had counted almost one million eggs, and logged about 3,000 hours.
Expecting that genetic variation would greatly increase with age, Rose was surprised to find that the results were completely different: Genetic variation of fecundity in late life wasn’t greater than the variation at earlier ages. The results refuted Charlesworth’s “garbage-can” hypothesis and suggested that late life was not simply “a receptacle in which bad mutations accumulated.”
Rose tended this research while Charlesworth was on sabbatical in North Carolina, but he began to wonder—and wander. He recalled that, in late 1977, before Charlesworth’s initial hypothesis was disproved, he had come across an article by J.M. Wattiaux on the effect of parental age on the offspring produced at that age. Wattiaux was a French scientist working at the University of Leuven in Belgium. His study used the same fruit flies that Rose had been working with for Charlesworth’s study. Wattiaux found that when he made each new generation of fruit flies that were the offspring of old parents exclusively, the flies showed an increased life span after each generation. But Wattiaux didn’t know why his fruit flies lived longer. He felt that longevity increased because of a nongenetic effect, but he didn’t have any direct evidence.
Rose did. Wattiaux’s results, he saw, showed the importance of the force of natural selection. He believed that, because natural selection stops working at a late age and fails to eliminate genes with detrimental effects, these bad genes would not be removed by natural selection. Instead, they would accumulate. In populations that reproduce early, natural selection declines early. Alternatively, populations that are old when they reproduce will continue to be subject to powerful selection until they begin to reproduce. Thus, by allowing older flies to reproduce over generations, natural selection would continue to choose the flies that are able to breed at a later age—the fittest flies.
Why are these experiments conducted with fruit flies, of all lab animals? Better yet, if scientists can prolong the lifespan of Drosophila, what does this mean for humans? Since TH Morgan’s groundbreaking work in the early 20th century, fruit flies have been instrumental in lab research. They are fecund and, even more important, have conveniently short life spans. Delayed reproduction in humans would surely take too many years to accurately observe because of long human life span. Fruit fly generations are typically about two weeks long, making them perfect for multi-generational studies. Rose’s new study would only take a few years; natural selection could be used to postpone aging without time being problematic.
The breeding of these Methuselah flies (Rose’s term for animals with a substantially increased life span) would be what Rose claimed as “the single most important breakthrough in the long history of research on aging: the deliberate creation of longer-lived animals.”
In December 1977, weeks after reading Wattiaux, Rose began to breed the flies. Unbeknownst to his supervisor, who was still in America, he commenced his own experiment. He collected eggs from adult flies that were at least 35 days old—two weeks longer than the norm—and hatched their eggs. This delay of reproduction forced natural selection to favor the survival of adults for three more weeks, producing a “fitter” generation of flies.
Rose continued the late reproduction experiment for 12 generations, or about 60 weeks. In the spring of 1979, he analyzed the data and proudly presented his findings to Charlesworth: His Methuselah flies lived about 10 percent longer than the average fly. Prolonging the influence of natural selection could postpone aging.
In May 2001, he told Discover magazine, "That was the all-time most exciting hour of my scientific career," Rose says. "You could compare it to losing your virginity or getting married—the first time.”
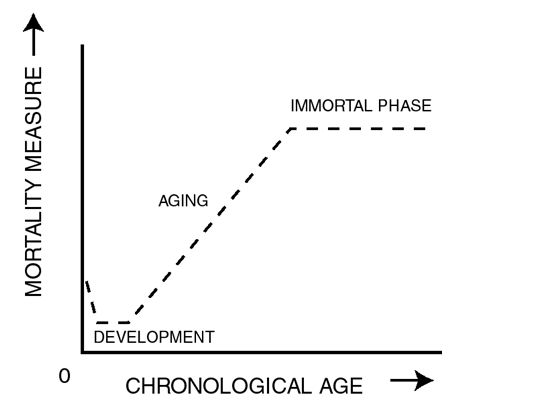
Biological Immortality in Late Life (credit: Michael R. Rose, Laurence Mueller)
A plethora of experiments, articles, and books later, Rose is a professor of evolutionary biology at University of California, Irvine. He is still in pursuit of immortality, speaking frequently and ardently about his findings and what they mean for the future. His famed Methuselah flies demonstrated that aging is a process that occurs in organisms via evolution by natural selection. Dr. Laurence Mueller, a longtime colleague of Rose, explained. “Now that we understand that natural selection results in the cessation of aging, then it’s not a biological impossibility to perhaps manipulate this process to a point which is beneficial to individuals.”
Mueller and Rose, who have known each other since 1983, share a wall between their offices on the fourth floor of UC Irvine’s Steinhaus Hall, where I had been counting fruit flies with Marta. “We share a brain,” Mueller said of Rose. Tall and tan, with dark grey combed back hair and blue jeans, Mueller looks less like he’s spent years of his life toiling away in a lab and more like someone who has been captaining a yacht off some faraway coast. “Depending on people you talk to, some people think, ‘Well, you know what? In Western societies, we already live to be pretty old. Maybe we really shouldn’t be trying to make us live any longer.’” He chuckled. “We’ve got way too many people, and, you know, some could argue about that. But like I said, the goal isn’t to have you hooked up to a life support machine for 100 years. No one’s interested in that. It really is to have the characteristics of a youthful person for a more prolonged period of time.”
Rose has already accomplished this with his flies.
“If you can understand how those long-lived flies differ from their normal counterparts, then the idea is: Can that understanding then lead to the intervention scheme where we could, through a drug or some other manipulation of our bodies basically create an environment within us that does the same thing that these altered genetics do for the flies?
“Without having to change our genes, we might be able to change the environment of our bodies so that we can live longer.”
Last December, Rose spoke at a wide-ranging conference at Caltech called Humanity Plus, but he was not talking fruit flies. He was talking about humans, about “Building Methuselahs,” and about, as the conference session was named, “Radically Increasing the Human Healthspan.” Rose, now in his fifties and bespectacled with graying hair, seemed at ease lecturing to his colleagues at the conference. He resembled the archetypal professor as he spoke about his life’s work, yet looked young for his age. In a dark suit and white collared shirt, Rose gave an overview of his well-known research with fruit flies. He presented a graph (see above) that Mueller created for a study from the 1990s; it demonstrated how mortality rates increase exponentially in a population and then level off, creating an “immortality plateau.”
This leveling-off occurs when a species reaches a state where it ceases to age, meaning that it doesn’t experience any more loss of physiological function. This is evident in humans as well as Drosophila: Mortality rates increase until a certain point in late life—90, for instance—and then stay the same. In essence, the body ages less dramatically after 90 than between 80 and 90.
If this plateau could be engineered to begin earlier (that is, if the cessation of aging could begin earlier), humans could have an indefinite life span.
At Caltech, Rose began to stray from his usual PowerPoint. The subject of his talk, he said, was something he dubbed the “Natural Immortality Technology.” This “isn’t simple or easy, but it’s free,” he told the audience, “and you can start it this evening.” The technology—really a lifestyle regimen linked to future technical breakthroughs—centers around what is “natural” for humans. He went on: Nature, rather than today’s chaotic, technology-dependent lifestyle, makes up our true evolutionary context.
As humans, what is our ideal environment? “Not the industrial lifestyle with lots of sugar, lots of immobility, lots of stress, watching news programs, having your stock portfolio get fried by psychopaths on Wall Street. None of that is good for your health or survival—at any age.”
Instead, Rose advocates a diet of food that our ancestors were accustomed to thousands of years ago. Often referred to as the “Paleolithic diet”, it includes meat, fish, vegetables, fruits and nuts—food that’s not often a part of today’s preservative-ridden, over-processed cuisine. Winding back the clock back even more, the invention of agriculture alone isn’t part of human nature as biology intended it. For most of our existence, humans have had a diet that consists of natural foods (hence the “Caveman diet” nickname). According to Rose, natural selection has favored physiological adaptations that help us process those types of foods without developing diseases or disorders from them.
But within the last 10,000 years or so, we’ve shifted to an agricultural diet that includes milk and grains. Although 10,000 years isn’t very long relative to the span of human existence, we’ve had a few generations that were able to adapt to this “new” diet. Said Mueller, “Using this very general theory, our inference should be that natural selection works strongly to have young people adapt to this new diet and not have severe reactions to it, but because natural selection works so weakly when we’re old, it actually may not have had enough time to allow us older people to adapt to the agricultural diet.” It may be beneficial for us, as we get older, to follow a diet that’s more consistent with the diet to which our ancestors adapted.
A diet that rules out the instant gratification of fast food and the guilty pleasure of junk food and encourages more fresh fruits and vegetables would be beneficial, but Rose’s Paleo diet is based on more than just a reduction of caloric intake. It’s simply how natural selection works.
“It may turn out that once natural selection makes a young person able to tolerate rice or wheat, that adaptation carries throughout your life, but if in fact there’s some age-specificity to your ability to tolerate that stuff, then maybe it’s not your case,” said Mueller. As children, we’re all raised on milk, as humans have been since the caveman days. Unlike us, however, our ancestors never had milk after they were weaned from their mothers.
Consequently, modern humans have evolved such that many of us lose the ability to digest milk as we get older. Rose’s natural immortality plan is a way that we can apply the knowledge gained from his and Rose’s research on aging to change current habits to benefit people today.
“Really, the diet is just the absence of the food products that are basically a novel invention of modern agriculture, however you define those. Twinkies, all that stuff,” said Mueller.
Is he about to start cooking like a caveman? “Although I believe in it, I love cheese too much. I love pasta.”
Rose’s “Recipe for Natural Immortality” begins with adopting the diet of a hunter-gatherer when you turn 35. The next step is to use the best of modern medicine to get you to the immortality plateau. Then, as time passes and technology continues to advance and becomes more available, use autologous tissue repair (a type of repair in which tissue is derived from your own body). This type of tissue replacement, used for the generation of skin graft tissue, corneal cells, heart muscle, and more, isn’t something that Rose personally works on, but is imperative to incorporate this therapy in a venture towards immortality. Think of the body like a vintage car. If you replace certain parts as the years go by, it can keep running indefinitely—a well-oiled machine. Finally, Rose advocates for “new-generation pharmaceuticals” as they become available in about ten years.
What sounds like the grounds for a new diet book may seem fairly disconnected to Rose’s fruit flies. But Rose insists it is the latest manifestation of his and Mueller’s constantly evolving research, all based on evidence gathered from the diminutive yet immensely dependable Drosophila.
In 2011, Rose is not as odd a duck as he might have been even ten years ago. Scorned by establishment science in years past, longevity research has been taken up by a new wave of bio-gerontologists. What was once deemed pseudo-scientific drivel has now, due to developments by scientists like Charlesworth and Rose and giant shifts in the demographics of aging, become a hot topic. Aubrey de Grey, founder of the SENS Foundation (Strategies for Engineering Negligible Senescence) has known Rose for 11 years. While they have never worked together, they are both leading voices in the field of aging research. De Grey also spoke during Humanity Plus’ “Radically Increasing the Human Healthspan” session and argued that Rose’s statements about late life were overly simplified. The two could not be more different: Rose was clad in a dark suit and lectured from a Power Point; De Grey scribbled diagrams on a chalkboard wearing blue jeans and tennis shoes.
While Rose believes that humans can reach a plateau of biological immortality where personal death odds become constant, De Grey is convinced that a large percent of aging is due to accumulated damage in the body. Personal death odds increase as damage accumulates. Rather than stopping aging at an earlier age and maintaining the properties of middle-aged person, De Grey thinks that aging, as a product of accumulating damage, can happen and then be repaired. The exponential curve that Rose’s studies have demonstrated to level off at the end are inaccurate, said De Grey. The curve actually levels off, rises, and then levels off again. He believes that we cannot tell whether an immortality curve actually exists because we don’t have a direct way to tell how each individual’s risk of mortality is changing during life.
When I meet Rose for the first time, he responds to the criticism like he’s heard it hundreds of times. (That’s because he has.) There are two issues that people have with his work, he told me from behind a desk covered in stacks piled high with magazines and file folders. The first issue is with his science. “The second one is what people do with their own lives, so it’s sort of the academic question versus the practical question, or the biology question versus the medicine question,” Rose said. “Plenty of scientists disagree with my work. My work is scientifically controversial, not because people find anything wrong with it, but usually because it kind of blows their minds. It just seems like it can’t be true.” He takes scientific challenges to his work, like those by De Grey, in stride, but the other criticisms hit a nerve.
“You have all the ethical questions in terms of the practical and medical issue, ‘Should people live forever?’ and so on,” said Rose. “There, I should be clear and say if people want to die early, if people want to die at 72, that’s fine. It’s their personal choice. I don’t try to dictate to them what to do with their lives.”
Rose is known for being outspoken about people who disagree with his work on immortality based on religion. He told Discover, “I have on a number of occasions heard people give very moving addresses as to why we should die as soon as possible. I think the phrase that most stuck in my mind was, ‘So that we can know God’s love sooner.’ And let me just say for the record, I am all for those people dying. They can go ahead. I just know other people who don’t want to die, and least of all by the horrible and unattractive process of aging, and I don’t see any reason why they shouldn’t be allowed to go on living.”
His distaste for aging wasn’t what fueled his desire to find the key to immortality. On that sunny afternoon in his office, Rose said, “I started this work and I had no interest in aging at all. I had no interest in living longer myself and I had no concern about other people around me living longer. So it was only over time that my point of view changed.” From the first Drosophila experiment working under Charlesworth, Rose’s life has revolved around his research, leading to great triumphs as well as setbacks, and, as he reveals in The Long Tomorrow, personal sacrifice.
In 1977, Rose was working on his 3,000-hour experiment for Charlesworth. It was then, early in his career, that Rose experienced the tragedy of a loved one who certainly met death too early by anyone’s standards: his younger brother Tim had hanged himself in some woods in Ontario. As he came to terms with his brother’s death, (“sheltered by distance, and by my research, from my family’s trauma,” he wrote), he read more scientific literature and began to have new ideas for experiments. This led him to the delayed reproduction experiment that would change his career.
Three years later, Rose was faced with a crisis in his marriage. His wife, Frances Wilson, was devastated by the murder of a member of her own family, her stepbrother, Eric. He had been murdered by hitchhikers in the same van that Rose and Wilson had taken on their honeymoon years earlier. As Rose tells it, the news of this death, as well as the suicide of Rose’s brother, left his wife in emotional disrepair.
The demands of Rose’s profession strained their relationship further. Rose became depressed and felt that his marriage was barely surviving. In 1980, Rose was offered a faculty position at Dalhousie University in Halifax. He hoped that the return to their native country could repair their relationship, but it didn’t, and they legally separated in 1982. Wilson moved back to Ontario and Rose continued to teach. He was on a series of seminars along the West Coast in 1983 and was preparing to speak at an afternoon seminar at the University of Washington when a police officer asked for him. The officer told Rose that his wife had killed herself in her apartment. Rose’s depression continued for some time, he wrote, but did not last. “I sprang back into action with a determination that would dominate the next decade of my life.” His output of publications soared that year and, as of 2011, continue uninterrupted. “My personal relationships have not fared as well… My work has been my shelter from life.”
By 1984, wrote Rose, he was dedicated to his work on aging. He’d been hesitant in the beginning of his career, but now he was certain that he would pursue his research on aging and immortality. “Death had made me less of a dilettante about aging,” wrote Rose. “I had made its acquaintance in a very personal way, and I did not find it an engaging companion. Let the Christian moralists swoon in its chilly embrace. I find their graveyard desires revolting.”
While my time with Rose was brief, seeing his students hunched over boxes of Drosophila in his lab was, for me, a surreal vision of one man’s legendary work. I had a glimpse into his fascinating world, where long hours peering over tiny fruit flies lead to a journey in the extension of human life span. On the way out that day, I noticed a yellowed, dog-eared poster above the exit. In fading black marker it read, “Welcome to Rose Lab… Welcome to the lab that never ends.”
Louise Lao is a graduating senior in Literary Journalism and Art History at the University of California, Irvine. Her work has appeared in the San Mateo Daily Journal and in New University, UCI’s student newspaper. She is currently interning in the fashion industry at a Los Angeles label and aspires to create her own line in the future. This is her first article for Science 2.0





Comments