A decades-old cancer mystery has been solved by researchers at Cold Spring Harbor Laboratory (CSHL). "We not only found a critical tumor suppressor gene, but have revealed a master switch for a tumor suppressive network that means more targeted and effective cancer therapy in the future," said CSHL Associate Professor Alea Mills, Ph.D. The study, headed by Mills, was published in the February issue of Cell.
Specifically, Mills' discovery identifies CHD5, a protein that prevents cancer, as a novel tumor suppressor, mapping to a specific portion of chromosome 1 known as 1p36. When CHD5 is not doing its job, the machinery within our cells that normally prevents cancer is switched off. The ability of CHD5 to function as a master switch for a tumor suppressive network suggests that this gene is responsible for a large number of diverse forms of human cancers. "CHD5 functions like a circuit breaker that regulates the tumor-preventing power in our cells—when it blows, cancer occurs," explains Mills. Modulation of CHD5 activity may provide novel strategies for better design of more effective cancer therapies. This gene has remained a mystery until the discovery by Mills' team.
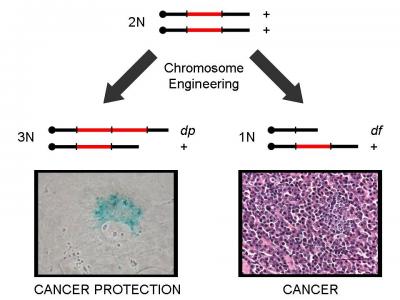
After they located the region where the tumor suppressor resided, the Mills team sought to identify which genes in that area were responsible for tumor suppression. Their results showed that reducing expression of a single gene--CHD5--made cells that had been rendered slow growing by adding an extra copy of the region, grow like normal cells.
The findings of Mills' study will influence the future of cancer research. It shows that deletion of a part of 1p36 causes cancer and increased "dosage" of CHD5 triggers extra tumor suppression. One extra dose, or copy, caused cells to either stop dividing or to undergo cell suicide by switching on a battery of potent tumor protective machinery. This work indicates that pharmaceuticals that switch on CHD5 may provide a way to treat many types of human cancer.
The research team worked with mouse models which enabled them to investigate the gains and losses of the chromosome segment corresponding to human 1p36. To extend the research to human cancer, Mills collaborated with Stanford University researchers Dr. Hannes Vogel and Dr. Markus Bredel to study whether CHD5 also functioned as a tumor suppressor in humans. They discovered that glioma, a specific form of brain tumor, frequently had deletion of CHD5, demonstrating the important role of CHD5 in human cancer.
This research and discovery was funded largely by private sector donations which are important to support state-of-the-art research and discoveries that may not traditionally be funded by the government. "The advance by Dr. Mills and her team shows that CSHL's strategy of providing financial resources to outstanding young scientists pays off towards diagnosing and treating disease and human suffering," states CSHL President Bruce Stillman.
Written from a news release issued by Cold Spring Harbor Laboratory





Comments