Reducing the expression of a pair of proteins known as NEETs, NAF-1 and mitoNEET, significantly reduced cancer cell proliferation and breast cancer tumor size, according to a new paper.
NEET proteins transport iron molecules or iron sulfur clusters inside cells. The proteins naturally adhere to the outer surface of the mitochondria, the "power plant" that supplies cells with chemical energy. Mitochondria also play a role in a cell's life cycle, including its death.
The new research stemmed from a recent CTBP study of the shape and functions of one of the proteins, mitoNEET. The other protein, NAF-1, is closely related to mitoNEET.
Co-author José Onuchic of Rice University's Center for Theoretical Biological Physics (CTBP), said the new study was triggered by the team's recognition of a connection between NEETs and reduced rates of breast cancer among women who take a diabetes drug that targets mitoNEET. "NEET proteins play a key role in the overall stress response of cells. Any time you stress a system, these proteins are there to help, but in cases where cells are overcome by stress, NEETs can become highly overexpressed. That's what drew our interest in a potential connection to cancer."
"Maintaining mitochondrial health is a key factor in the health of a cell," said co-author Rachel Nechushtai, a professor at the Alexander Siberman Life Science Institute of the Hebrew University of Jerusalem. She and Ron Mittler, professor of biological sciences at University of North Texas, Denton (UNT), are experts in iron and reactive oxygen metabolism and collaborated on a prior study that identified a key role for NEET proteins in maintaining mitochondrial function in cells under conditions that resembled cancer cell metabolism.
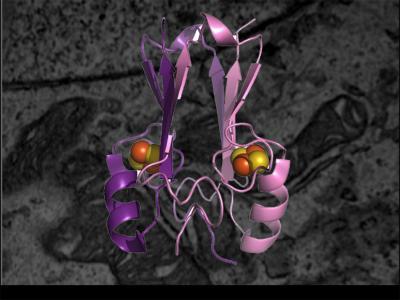
Enlarged illustration of a mitoNEET protein backbone fold (pink and purple) with essential iron-sulfur clusters (orange and yellow) is superimposed on a microscopic image of a breast cancer cell's mitochondria. Credit: P. Jennings/UCSD
"We found that novel motions in NEET proteins control cluster properties and suggest the NEET proteins can be your best friend or worst enemy," said Patricia Jennings, professor of chemistry and biochemistry at UCSD.
Experiments in Mittler's and Nechushtai's labs found an overabundance of both mitoNEET and NAF-1 in breast cancer cells. Moreover, the team found a direct correlation between NEET protein levels and the overall progression of the disease.
The results suggest that the overabundance of NEETs is a factor in the runaway growth of cancer cells, and that would make NEETs a prime target for anticancer drugs.
"Developing drugs that target important cellular pathways that are specific for cancer cells is perhaps the most important goal of anticancer therapy," Mittler said. "Historically, mitochondrial pathways have largely been overlooked, but our results indicate that these are important pathways and that targeting the NEET proteins could have a significant effect."
Jennings and Nechushtai first detailed the molecular structure of mitoNEET in 2007. They were also the first to analyze the unique structural features of NAF-1 three years ago. That work led to the realization that NAF-1 and mitoNEET had similar structures. But it also showed the two had distinct surface features, which means each interacts with a different set of partner proteins and can be individually targeted with drugs.
"What's interesting about these two proteins is that mitoNEET is always on the surface of the mitochondria membrane, while NAF-1 is on the mitochondria and the endoplasmic reticulum," Onuchic said. "A lot of messaging rolls between these two proteins. You have to affect both of them to stop proliferation."
Jennings said the researchers took the path less traveled in making their latest finding. "We work opposite from the way most people do," she said. "We start with the structure of a protein -- usually one with important properties but unknown function -- and we move into the biology from that point.
"In the case of the NEETs, we knew about the connection to diabetes, and Rachel and Ron's work showed the key regulation point involved iron and redox metabolism," Jennings said. "Ron's functional studies took us from the computational and cellular arena into the whole organism -- for example, in plants -- and confirmed that NEETs have an ancient role in iron and reactive oxygen metabolism."
Ideally, the researchers said, they would like to see drug designers create small molecules that are customized to target cancer cells and block NEETs from interacting with other proteins that are key players in chemotherapy drug resistance. There's also a chance that using this approach could make tumors more susceptible to standard chemotherapy.
"Your immune system is trained to build resistance to one attack, but not to a coordinated attack," Onuchic said.
Citation: Yang-Sung Sohn, Sagi Tamir, Luhua Song, Dorit Michaeli, Imad Matouk, Andrea R. Conlan, Yael Harir, Sarah H. Holt, Vladimir Shulaev, Mark L. Paddock, Abraham Hochberg, Ioav Z. Cabanchick, José N. Onuchic, Patricia A. Jennings, Rachel Nechushtai, and Ron Mittler, 'NAF-1 and mitoNEET are central to human breast cancer proliferation by maintaining mitochondrial homeostasis and promoting tumor growth', PNAS August 19, 2013, doi:10.1073/pnas.1313198110





Comments