Recently, a direct influence of excessive regional neuronal activity on Alzheimer pathology was found in animal experiments. By showing that highly connected 'hub' regions (which display most Alzheimer pathology) indeed possess the highest levels of activity, the present study offers support for the unconventional view that brain dynamics may play a causal role in Alzheimer. As first author, Willem de Haan, says, "this implies that the investigation of factors regulating neuronal activity may open up novel ways to detect, elucidate and counter the disease".
Using a computational model of the human cortex, the authors simulated progressive synaptic damage to brain regions based on their level of activity, and subsequently investigated the effect on the remaining network. The large-scale network consisted of 78 neural masses, connected according to a human DTI-based cortical topology. Spike density and spectral power were positively correlated with structural and functional node degrees, confirming the high activity of hub regions, also offering a possible explanation for high resting state Default Mode Network activity.
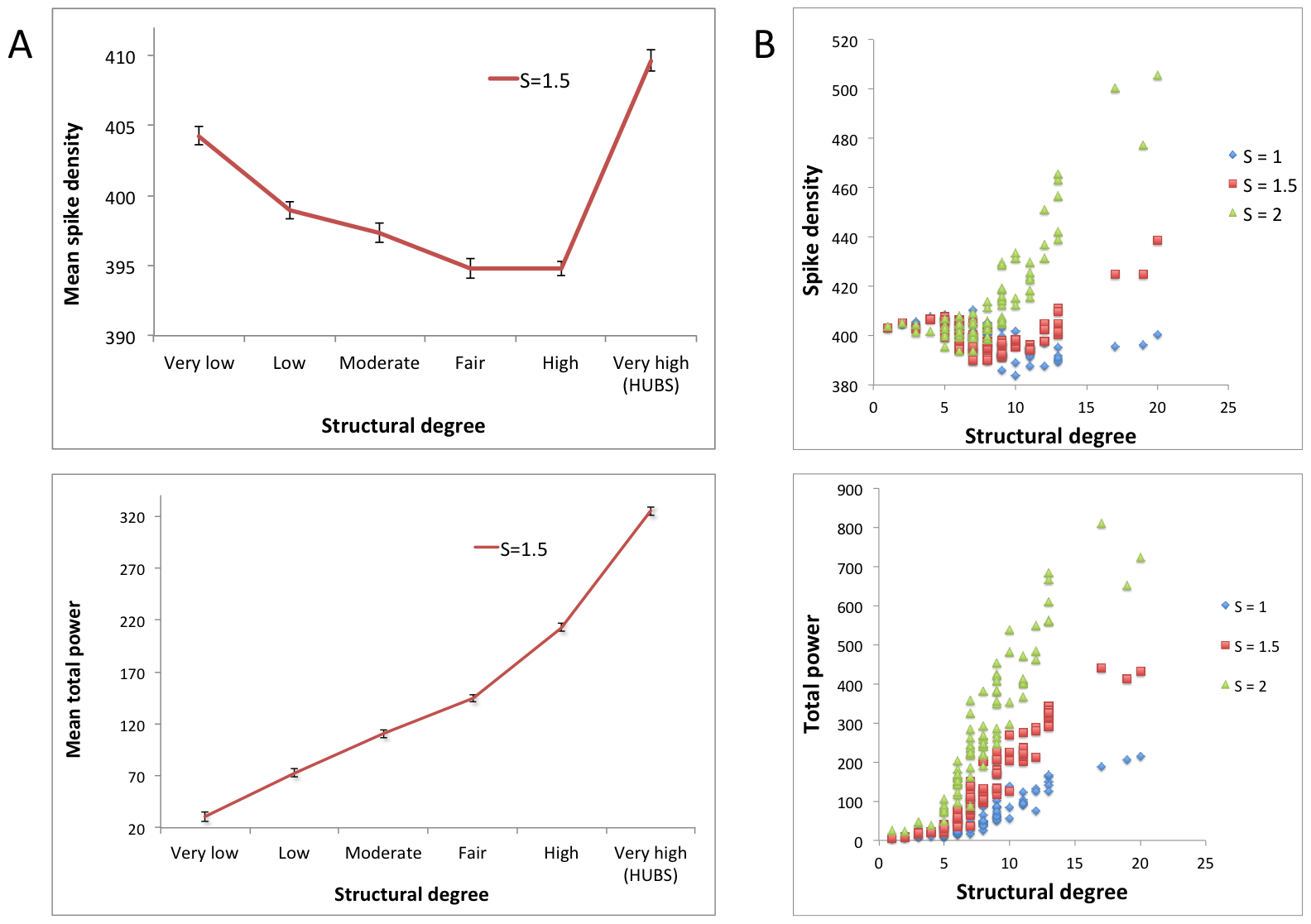
CLICK IMAGE FOR LARGER SIZE. Relation between structural degree and neuronal activity. A: Six bins with ascending mean structural degrees are plotted against their average spike density and total power values. Nodes in the ‘very high’ degree bin were defined as hubs. Coupling strength (S) between neural masses was set to 1.5. Error bars indicate standard deviation within each bin. B: Similar plots as in the left panel, but for every region individually, and for three different coupling strengths S (see Text S1, section 3). Source: doi:10.1371/journal.pcbi.1002582.g001 (link below)
With this 'activity dependent degeneration' model, they could not only offer an explanation for the distribution pattern of Alzheimer pathology but also reproduce a range of phenomena encountered in actual neurophysiological data of Alzheimer patients: loss and slowing of neuronal activity, loss of communication between areas, and specific changes in brain network organization.
In upcoming projects the authors plan to verify the predictions from this study in patient data, but also to continue modeling studies on ‘hub’ regions and this marked amyloid-β deposition at an early stage.
Citation: de Haan W, Mott K, van Straaten ECW, Scheltens P, Stam CJ (2012) Activity Dependent Degeneration Explains Hub Vulnerability in Alzheimer's Disease. PLoS Comput Biol 8(8): e1002582. doi:10.1371/journal.pcbi.1002582





Comments