That may be because it is hard to understand. But progress is being made. A group of researchers from the Andalusian Centre for Molecular Biology and Regenerative Medicine (CABIMER) has revealed new ways to understand the molecular basis of some human diseases that are stem from poor functioning of the mitochondria and, in this way, allow for the development of therapies against these diseases.
Eliminating any excess of defective products that might have accumulated in the mitochondria is vital, as its presence generates mitochondrial instability and information loss on the mitochondrial genome.
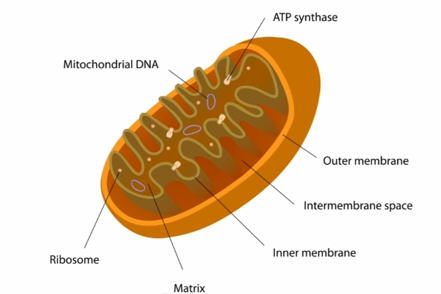
Mitochondria are organelles that exist within cells. They breathe the oxygen that comes to the cells to catabolise sugars and produce energy. They also make proteins as important as the Iron-Sulphur proteins with different functions that are essential for DNA metabolism, such as replication and repair of the DNA nucleus. Mitochondria have their own genome.
The loss of mitochondrial information and of mitochondria gives rise to defective cell metabolism. As well as the lack of capacity to generate the energy necessary for the cells, the loss of mitochondrial information can generate an increase in oxygen free radicals that attack and damage the genetic material or produce Iron-Sulphur protein deficiencies. All this brings about incorrect cell functioning and eventually cell death.
The study reveals that Degradasome, a complex of two proteins whose function is to eliminate the defective RNA that is produced in the mitochondria, is essential for maintaining mitochondrial genome integrity. If the Degradasome proteins are inactive, RNA accumulates in the mitochondria and forms abnormal structures, known as DNA-RNA hybrids, that are very harmful because when found in excess, they impede mitochondrial DNA replication. The consequence of this is that as the cells divide, they lose mitochondrial DNA, so compromising mitochondrial and cell function.
Further to the basic interest in this discovery, the study can bring new ways to understand the molecular basis of some mitochondrial diseases and help to develop therapies for different human diseases that stem from poor mitochondrial functioning. Although they can occur because of defects in mitochondrial proteins, they are also associated with the loss of mitochondrial genetic material and with the material of mitochondria. In summary, Degradasome, when it eliminates defective RNA, allows for the propagation of healthy mitochondria in the cells, so preventing possible mitochondrial diseases.





Comments