All of those combined have caused a real dearth in development of new antibiotics - but as mandated lower costs for drugs internationally show, if the United States does not develop them, no one will because the costs are not worthwhile without America. It is no secret antiobiotic resistance is the norm, evolution continues to occur and bacteria outlasted the dinosaurs and will probably outlast us also.
Now the governments that caused a shortage of new development are in a panic about it. Just throwing more money at a crisis is not a solution, though money and regulations are the only tools governments provide. Nor is risking lives by limiting antibiotics.
DOI: doi.org/10.1111/eva.12808 (open access)
Fitness Costs Are Unreliable Servants
In a new paper, Dr. Ben Raymond of the University of Exeter of the Centre for Ecology and Conversation outlines five rules for "sustainable use" of antibiotics. These include acting to protect new drugs before resistance becomes a problem, using more diverse antimicrobials to reduce long-term use of single drugs, and using data to design management plans for particular superbugs.
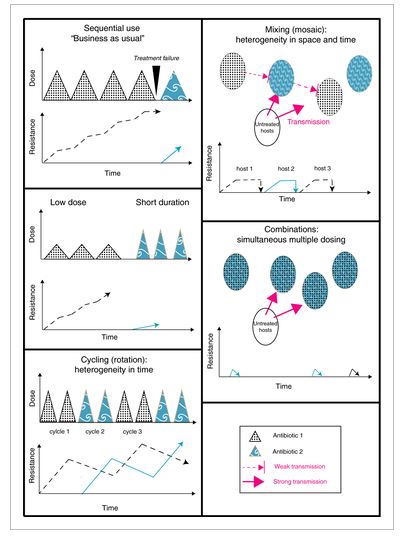
1. Prevention of resistance. Preventiong is not slathering children in antibacterial soaps, it is use of antibiotic “cycling” and “mixing” to slow natural selection in bacteria, which provides longer periods of time for new development to occur.
2. Don't rely on "fitness costs". Some plans depend on stopping use of a drug, in the hope that resistant bacteria suffer a "fitness cost" - dying out because they carry resistance genes that are no longer useful. This can work, but Dr Raymond warns that resistance to a drug does not necessarily go away just because use of that drug stops.
3. Limit supply of mutations. One way to do this is to use combinations of antibiotics, as microbes rarely develop resistance to multiple antibiotics at once. Dr Raymond also says it's "madness" from a resistance management perspective to build up a massive supply of resistance genes in the environment. Resistance in the environment can come from waste water and use of antibiotics in animals. "As an individual you are very unlikely to have acquired an antibiotic resistance microbe from an animal, but it's highly likely that environmental contamination has helped some of the microbes in your body acquire resistance," he said.
4. Low doses don't work, short courses might. A much greater pool of mutations can give microbes resistance to low doses of antibiotics, so such doses might help resistance evolve. Short, intensive courses of antibiotics might help patients without giving microbes the opportunity to evolve.
5. Information is power. "If you don't know what kind of resistance is around among patients or in your hospital, you could give people the wrong drug at the wrong time," said Dr Raymond. "The more data you have, the better you can design your resistance management programs. Resistance management programs should target specific microbes or groups of microbes, rather than resistance in general.
"Some humility in the face of natural selection can ensure that human creativity keeps pace with evolutionary innovation."





Comments