Radioactive decay
To start off with, here is a little recap of the physics that is important. Atoms can be thought of as made up of protons, electrons and neutrons. The type of element is determined by the number of protons. The number of protons in any element is fixed, but the number of neutrons is variable (within limits). When you get 2 atoms of an element, with a different number of neutrons, they are known as isotopes of that element. The number of neutrons affects the stability of the atom and for every naturally occurring atom, there is a optimal number (or range of numbers) of neutrons needed to keep the atom stable.
During fission reactions (the type of reactions in all commercial nuclear power stations), unstable isotopes of an element are created from the splitting of an atom. These unstable isotopes will eventually decay via one of various decay processes. Three of these processes are important for our discussion: alpha, beta and gamma decay.
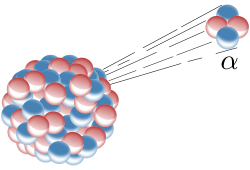
During alpha decay, the unstable atom emits a helium nuclei (composed of 2 neutrons and 2 protons) from the atom.
There are 2 types of Beta decay (Beta- and Beta+).
-
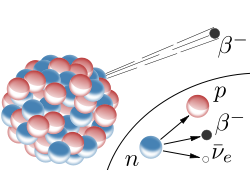 Beta- decay occurs for isotopes with an excess of neutrons. During this decay, a neutron is converted into a proton (thereby changing the element) while releasing an electron and another elementary particle known as a neutrino. (N.B. The neutrino is largely irrelevant for this discussion due to the fact that they (for all practical purposes) do not interact with everyday matter.)
Beta- decay occurs for isotopes with an excess of neutrons. During this decay, a neutron is converted into a proton (thereby changing the element) while releasing an electron and another elementary particle known as a neutrino. (N.B. The neutrino is largely irrelevant for this discussion due to the fact that they (for all practical purposes) do not interact with everyday matter.) - Beta+ decay can occur if there are too few neutrons in the nucleus. In this case, a proton is converted into a neutron while emitting a positron (same as a electron except that the charge is positive instead of negative) and a neutrino. Beta+ decay can only occur if the atom has enough energy to overcome the mass difference between an proton and a neutron. (If the atom doesn't have enough energy, a process known as electron capture occurs where a proton captures an electron to become a neutron while emitting a neutrino - this process doesn't emit anything (except the neutrino which is irrelevant) and therefore cannot be considered a form of atomic decay.)
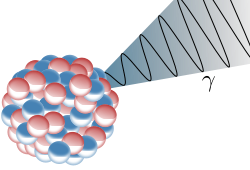
The last form of radioactive decay to be discussed here is gamma decay. Generally this type of decay will accompany an alpha or a beta decay. After an alpha or beta decay, the remaining atom can be in an excited state. In this situation, the atom can radiate a high energy photon to lose some of the excess energy that it possesses.
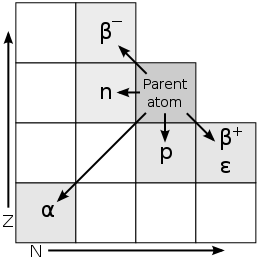
(Added during editing) During the alpha and beta forms of decay described above, the parent element will change to another element. The resulting atom can easily be determined by identifying the remaining number of protons and neutrons. The following graphic clearly depicts the outcome of these 2 types of radiation as well as the atomic changes that occur when an atom loses a proton (p) or a neutron(n). A change in the row means that a change in element.
Ionizing radiation
Ok, now that most of the relevant nuclear physics is covered, lets move on to another important concept. Radiation is known as ionizing, if when it interacts with an atom, it creates an ion (a charged particle). This occurs when the emitted radiation detaches electrons from another atom or molecule.
Whether or not radiation will be ionizing is dependent on the energy of the radiation. In other words, a single particle or photon of radiation must posses a certain amount of energy before it is able to ionize an atom or molecule, the cumulative amount of energy is irrelevant.
Ionizing radiation is important because the resultant charged molecule or atom (aka free radicals) is chemically quite reactive. If the radiation has ionized a molecule within living organism, then the resulting free radicals can damage the DNA within a cell. In addition to this, Ionizing radiation can directly damage the DNA by ionizing it or by literally breaking through the bonds of the DNA molecule.
It is clear then that being exposed to large amounts of ionizing radiation is detrimental to health.
The Lingo
Now that we know the types of radiation and how it can potentially effect us, let us try and work through the different terms that is common when dealing with radiation. Most of us think we know exactly what it all means, but the reality is a bit different. The following are the most important terms to know:
Absorbed dose: this refers to the total energy that has been deposited into a certain mass of an item - measured in units called grays.
While this measure is important, it is an incomplete measure of the effect that a certain amount of radiation will have on a biological entity. Due to the fact that a minimum amount of energy is required for ionization, a photon will not be able to affect a cell in the same way that an alpha particle of the same energy would. As a general principle, the heavier the particle, the more biologically damaging it is. To cater for this, weighting factors are added to this measure so that a more synthetic, but more biologically significant measure of radiation is identified.
Equivalent dose: this refers to the absorbed dose weighted by a weighting factor dependent on the mass of the particle and it's energy - measured in a unit called sieverts. A weighting factor table can be found in Wikipedia's "Equivalent dose" entry (http://en.wikipedia.org/wiki/Equivalent_dose)
The earth naturally exposes us to radiation, and while the cumulative dose has its value, the more prudent measure would be the absorbed dose rate and equivalent dose rate.
(NB. What gets reported in the the media is the Equivalent dose rate. Well if you ask, how do they identify the type of particle depositing the energy so that you can multiply the weighting factor, the simple answer is that they don't. Luckily the radioactive material that is released into the environment due to the venting of steam and other non-catastrophic releases, decays using beta and gamma decay. The weighting factor for these 2 types of radiation is 1 which means that the total energy absorbed is equal to the equivalent dose. The instruments used to measure them are actually measuring the total energy)
Geiger counter: this is the device that gets used to measure the energy of ionizing radiation in an area. This device is a useful portable device and can measure beta and gamma radiation quite well.
Half-life: this refers to the time taken for half of a certain isotope to decay. It is impossible to predict when an individual atom of an isotope will decay, but the half-life can be used to determine approximately how many atoms of an isotope can decay in a certain time period - which in turn allows us to know how much energy is released in that time period due to the decay.
Biological half-life: This is not a very commonly used term outside of the medical industry, but it is important none the less. The Biological half-life refers to the amount of time it will take for about half of a certain substance to be flushed out of a human's system. The substance can be a drug, radioactive substance or any other consumed substance.
The reader should now have sufficient knowledge about this topic to understand what typically can occur when exposed to radiation. This will be the topic of the next section.
Direct exposure from the outside
As mentioned, the heavier the decay particle, the more effect it will have on biological cells. As such alpha particles will have a greater effect on an individual than lighter photons or gamma particles. Alpha particles are generally only released by large heavy atoms. These large atoms, when they are produced in a nuclear reactor, are trapped within the fuel pellet of a reactor and will not be transported outside of the fuel unless the fuel is severely damaged and the containment is completely breached. Because of this almost all of the alpha particles released will be in around the fuel rods. Alpha particles can however be easily stopped by something as thin as a sheet of paper and are therefore not an extremely dangerous threat.
Beta particles are a lot lighter than alpha particles and therefore their effects are not as severe. However, these particles are more difficult to shield against than alpha particles. Without aluminium shielding, Beta particles can penetrate the skin of a person and ionize molecules within his body.
Gamma particles are high energy photons. These particles are the most difficult to shield against, and requires several inches of lead or concrete to protect against them completely. There will be a constant barrage of gamma particles around exposed spent fuel rods and this is one of the reasons why there will be severe problems if a worker is exposed to a spent fuel rod directly. These concentration of gamma particles will decrease as the distance from the source increases and therefore the highest risk will be closest to the reactor.
Radioactive particles released to the environment
Radioactive isotopes can be broadly categorized into short-lived, medium-lived and long-lived isotopes. While there is no strict definition of what constitutes any category, these categories can be used to identify an important characteristic of radiation.
As was indicated previously, the rate at which energy is released is more important than total energy released. And due to the facts that short lived isotopes have very small half-lives, there will be an initially high rate of radioactive exposure from the decay of these particles. However, because these particles have such short half-lives, their larger environmental consequences are minimal i.e. they might kill everything in sight quickly, but it will be safe for people to live there almost immediately afterwards. (The large spikes in radiation during a nuclear accident are caused by short-lived isotopes)
The long-lived isotopes are a curious bunch. The energy released is distributed over such a large period of time that their actual biological effects are difficult to ascertain. In other words, your great-grandchild could get a tumor, but the statistical probability of that happening would be equivalent to anyone else's great-grandchild getting a tumor.
Now, the so called medium-lived isotopes are a little bit more problematic than the short or long lived isotopes. These isotopes do not decay very quickly, but do not remain stable long enough as to make its effects indistinguishable from the environment. So in other words, its effects might kill you and you probably wouldn't be able to move to an area affected by it for quite a significant amount of time (in human terms).
So are all the panic buttons ringing now.... hold on for a second. Over 99% of all fission products are retained in the fuel cells. In normal operation. there are virtually no radioactive isotopes escaping the fuel cell. The water used to cool a reactor under normal operations is de-mineralized water and therefore contains virtually no impurities. The neutrons, beta and gamma particles reaching the water does not activate any of the molecules in the water, and it remains safe. In emergency conditions where water with impurities are used (such as seawater) the beta and gamma particles together with some neutrons will slightly activate (make radioactive) the water. However, if the fuel rods are damaged or cracked, then the fission products could mix with the water making it radioactive. If this fuel is then vented to relieve pressure, then these radioactive isotopes will be released to the environment. A certain amount of fission products can also escape from exposed fuel rods if they are damaged.
If fission products are released into the environment, then they are free to interact with natural processes like the hydrological cycle and become part of the "chain of life". The larger effect of even modest releases of radioactive materials into the atmosphere will generally be dispersed and would be unlikely to cause above-normal rates of disease. If catastrophic release occurs, like in the case of Chernobyl, where almost all the fission products were released into the environment, then significant measurable consequences will occur.
Main isotopes to watch out for
While there are countless fission products being produced in a reactor, not all of these products will cause the same amount of damage to humans. Many of the fission products can be avoided by staying indoors and by thoroughly cleaning yourself if you are exposed. Many others will be inhaled as you move about but have such short biological half-lives (a measure of the time the isotope will stay in your body) that they will not cause serious health risks to the public. There are 3 main isotopes that could be potentially harmful:
Iodine-131: This is a short-lived radioactive element. It can easily enter your body since your thyroid gland can easily absorb Iodine from the environment. Iodine-131 has a half-life just over 8 days and can cause significant damage to cells by Beta decay. Absorbing Iodine-131 will increase the risk of thyroid cancer. Potassium Iodide capsules can be taken to prevent the body from absorbing the radioactive Iodine. The Potassium Iodide saturates your body with Iodine preventing further absorption. The capsules must be taken daily for as long as a significant threat exists.
Caesium-137: This is a medium-lived radioactive element. It is a soluble toxic element that has a half-life of about 30 years. Within a human body, it has a biological half life of around 70 days, but untreated, could cause significant damage if the equivalent dose is high enough. Caesium will biologically act similar to potassium but cannot be absorbed through inhalation. If accidental ingestion does occur, Caesium can be treated with the Chemical Prussian blue - a dye with some medical uses.
Strontium-90: This is also a medium-lived radioactive isotope with a half life of around 29 years. It can behave similar to Calcium. It usually enters the body through drinking or eating contaminated foods and drinks. It will attach itself to bones and can cause cancer if the dose is high enough.
Biological Effects
Well now that you know all of this, what does this mean to us? Well - once the DNA in a cell is damaged, it is not the end for us. Not by any long shot. DNA is an interesting chemical in that it has the peculiar ability to be able to repair itself. Given that, the following scenarios could occur:
- The DNA in the cell is damaged but it is able to repair itself
- The DNA in the cell is damaged and cannot repair itself - the automatic cell death occurs
- The DNA in the cell is damaged, it cannot repair itself, and has damaged the automatic cell death mechanism - the cell continues it's function in a damaged way, but cannot propagate the damaged genes
- The DNA in the cell is damaged, it cannot repair itself, has damaged the automatic cell death mechanism, the cell continues it's function in a damaged way, it propagate the damaged genes, but does not cause a malignant tumor
- The DNA in the cell is damaged, it cannot repair itself, has damaged the automatic cell death mechanism, the cell continues it's function in a damaged way, it propagate the damaged genes, and cannot help but form a malignant tumor
Cells have developed these capabilities through evolution as a means to survive cell damage. In fact, one should not be constrained to thinking that DNA damage is only caused by radiation. There are various reasons why free radicals can form within the body and damage DNA. Even a significantly strong heat source will damage cells.
(Added during editing) The following diagram puts the whole situation into perspective:
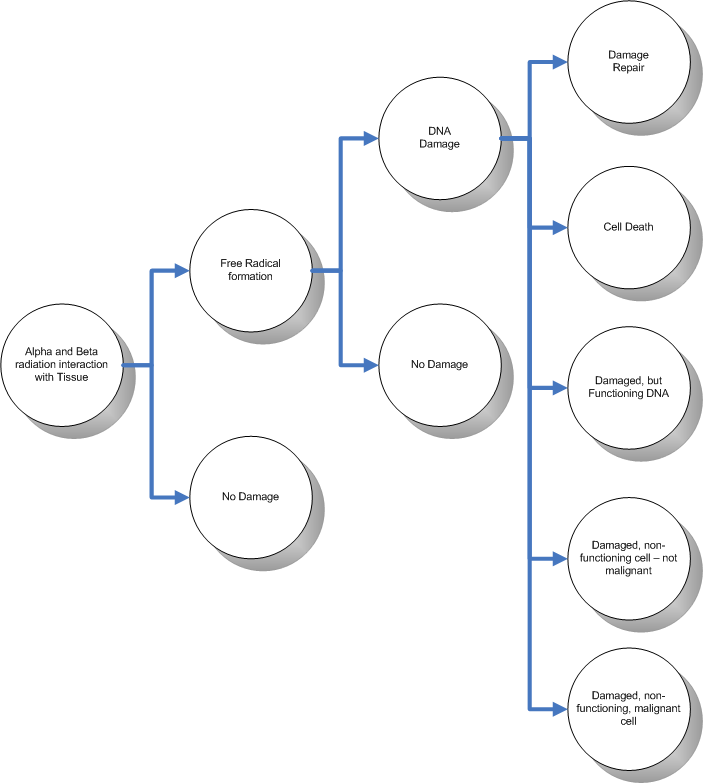
The various scenarios described above are largely dependent on the extent of the radiation exposure onto the cell. The higher the exposure, the more likely that the exposure will cause detrimental cell damage.
Doses and Risks
I hope this discussion has helped give readers a reasonable understanding of how radiation effects people in general. What is important to note is that the amount of radiation that is received is the key to determining what level of biological effect the radiation will have on the individual. The following list (extracted from wikipedia) will give an estimate of what the effects of a certain amount of cumulative radiation exposure within a short period will be.
A more detailed table can be found on Wikipedia's article on Acute Radiation Sickness. As a general rule of thumb, exposure should be limited to below 100mSv under normal conditions, and even in extreme circumstances no exposure above 1 Sv should be permitted. Once you exceed the 1 Sv level, effects will become increasingly severe.
- 0 – 0.25 Sv (0 - 250 mSv): None
- 0.25 – 1 Sv (250 - 1000 mSv): Some people feel nausea and loss of appetite; bone marrow, lymph nodes, spleen damaged.
- 1 – 3 Sv (1000 - 3000 mSv): Mild to severe nausea, loss of appetite, infection; more severe bone marrow, lymph node, spleen damage; recovery probable, not assured.
- 3 – 6 Sv (3000 - 6000 mSv): Severe nausea, loss of appetite; hemorrhaging, infection, diarrhea, peeling of skin, sterility; death if untreated.
- 6 – 10 Sv (6000 - 10000 mSv): Above symptoms plus central nervous system impairment; death expected.
- Above 10 Sv (10000 mSv): Incapacitation and death.
(The original article has been extended with clarifications and diagrams to enhance the reader's experience. Where additions have been made, these have been identified. Several grammar and spelling corrections were done and these have not been explicitly stated.)
Epilogue
As a final word, I'd like to say a few words about the events unfolding in Japan and to all of us reporting on the incident. The crew trying to control the reactors there are risking their lives to do what they must. Truly selfless individuals!
Shame on all of us who have tried to manipulate these events to suit our own agendas. Instead of leaving conjecture and speculation to the side we used this opportunity to nit pick each other's viewpoint. The outcome of these events will be change the outlook of the world, whether we want the change or not. But us bickering over nothings helps no one. I think we owe it to those hardworking men and women who are struggling to overcome these massive challenges to take a step back and just watch the work that they are doing. Leave the commentary for afterwards.




Comments