Their design exploits the unusual electrical properties of structures called single-wall carbon nanotubes, using them as "molecular wires in light harvesting cells," said Jong Hyun Choi, an assistant professor of mechanical engineering in the Birck Nanotechnology and Bindley Bioscience centers at Purdue's Discovery Park.
Photoelectrochemical cells convert sunlight into electricity and use an electrolyte, a liquid that conducts electricity, to transport electrons and create the current. The cells contain light-absorbing dyes called chromophores, chlorophyll-like molecules that degrade due to exposure to sunlight.
"The critical disadvantage of conventional photoelectrochemical cells is this degradation," Choi said, but if it works the new technology would overcome this problem just as nature does: by continuously replacing the photo-damaged dyes with new ones. "This sort of self-regeneration is done in plants every hour."
The new concept could make possible an innovative type of photoelectrochemical cell that continues operating at full capacity indefinitely, as long as new chromophores are added.
Findings were detailed during the International Mechanical Engineering Congress and Exhibition in Vancouver.
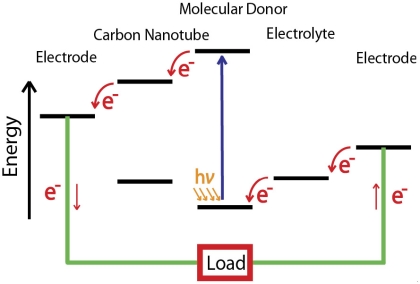
Mechanism of a photoelectrochemical cell incorporating carbon-nanotube nanohybrids. e−: Electrons. hν: Light energy.
The carbon nanotubes work as a platform to anchor strands of DNA. The DNA is engineered to have specific sequences of building blocks called nucleotides, enabling them to recognize and attach to the chromophores.
"The DNA recognizes the dye molecules, and then the system spontaneously self-assembles," Choi said
When the chromophores are ready to be replaced, they might be removed by using chemical processes or by adding new DNA strands with different nucleotide sequences, kicking off the damaged dye molecules. New chromophores would then be added.
Two elements are critical for the technology to mimic nature's self-repair mechanism: molecular recognition and thermodynamic metastability, or the ability of the system to continuously be dissolved and reassembled.
The research is an extension of earlier work that used biological chromophores taken from bacteria. Those indings were detailed in a research paper published in the November Nature Chemistry (http://www.nature.com/nchem/journal/v2/n11/abs/nchem.822.html).
However, using natural chromophores is difficult, and they must be harvested and isolated from bacteria, a process that would be expensive to reproduce on an industrial scale. "So instead of using biological chromophores, we want to use synthetic ones made of dyes called porphyrins," he said.
The talk and article were written by Choi, doctoral students Benjamin A. Baker and Tae-Gon Cha, and undergraduate students M. Dane Sauffer and Yujun Wu.





Comments