Scientists trying to investigate mechanisms at work in Alzheimer's and other neurodegenerative diseases have a new tool. A transgenic variety of zebrafish, which is transparent in the early stages of its life, called the "MitoFish" enables them to see how brain diseases disturb the transport of mitochondria, the power plants of the cell - within individual neurons of living animals.
Neurodegenerative diseases like Alzheimer's, Parkinson's and MS (multiple sclerosis) are different in their effects on patients' cognitive and motor functions, behavior, and prognosis - but on the level of individual neurons, common mechanisms can be observed that either cause or accompany nerve degeneration in a number of different diseases.
One of these is a disturbance in the transport of mitochondria, organelles that play several vital roles in the life of a cell — above all, delivering energy where it is needed. And in a neuron, an extremely power-hungry cell, that means moving mitochondria all the way down its longest extension, the axon. Studying mitochondria transport in other animal models of neurodegenerative disease, particularly in mice, has been revealing. But the MitoFish model opens up new possibilities.

A confocal image of a zebrafish head showing labeling of sensory axon membranes (yellow), mitochondria (cyan), and autofluorescence (red). Credit: Image: Leanne Godinho and Thomas Misgeld, TU Muenchen
The new model was jointly developed in the labs of Prof. Thomas Misgeld of the Technische Universität München (TUM) and Dr. Bettina Schmid, senior scientist of the German Center for Neurodegenerative Diseases (DZNE). "This collaboration has provided a system," Misgeld says, "with which we can try to understand the traffic rules or the life cycle of a given organelle, in this case mitochondria, in the context of a nerve cell that's existing in its physiological environment, where it is developing and changing. Most of these things we don't understand well enough to model them in another setting, so we have the organism do it for us."
The MitoFish is both readily manipulated, enabling researchers to pose specific questions, and literally transparent — allowing non-invasive in vivo observation of changes relevant to disease processes. It is possible to image a whole, living neuron over time and to follow the movements of mitochondria within it.
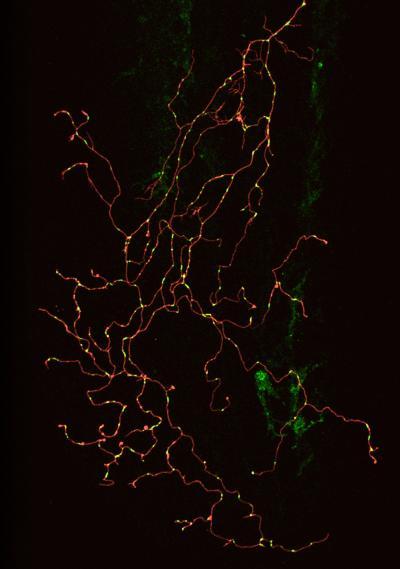
Confocal image of a single zebrafish sensory neuron with labeled membrane (red) and mitochondria (green). Image: Gabriela Plucinska, TU Muenchen
"The zebrafish is an established genetic model," Schmid explains, "which means you can bring foreign genes or certain proteins into a fish to test hypotheses about basic biology, disease mechanisms, or potential therapies. And because the early embryo is transparent, you can label specific nerve cells with a fluorescent protein and then look at them in an intact, living animal."
Misgeld's lab did imaging and analysis focused on organelle dynamics while Schmid's lab did the development of transgenic zebrafish models that are stable enough for long-term study.
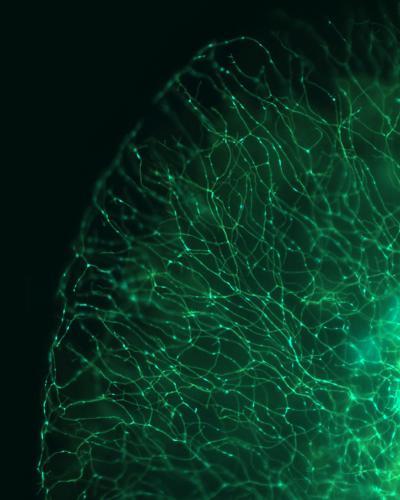
This is a wide-field image of a zebrafish tail showing labeling of sensory axon membranes (green) and mitochondria (cyan). Image: Dominik Paquet, LMU Munich
The driving force, they emphasize, is to understand more about Alzheimer's and other brain diseases to help steer the search for therapies in the right direction. "We need to understand how the machine works before we can operate it," Schmid says, "and modern biology is so technically advanced that no lab can be cutting-edge across the whole range of needed expertise."
Published in The Journal of Neuroscience





Comments