"All these gases are inert
Helium, neon, argon.
I’ll sing this song until it hurts
Krypton, xenon, radon." **
Helium, though, had only been suggested in 1868 when two astronomers (Janssen and Lockyer) had separately observed its spectral lines during a total solar eclipse. It was only in the 1890s that Sir William Ramsay discovered first argon, then krypton, neon, and xenon (meaning stranger), although argon had been observed by Cavendish in 1788 as a remnant of "phlogistated air" (nitrogen) which could not be absorbed into water by sparking with oxygen. Radon made itself apparent through radioactivity in 1896, observed as an element in 1900 and placed in the periodic table in 1903, in a chain of discovery involving many names.
All these gases were found not to react with any other elements. At first they were called rare gases, but soon acquired the names noble or inert gases. And with the discoveries of quantum mechanics, it became apparent that it was due to their filled outer electron shells that they would not react.
Well, not exactly. In 1962 Neil Bartlett of UBC-1 (University of British Columbia) published the preparation of xenon hexafluoroplatinate, and soon discovered many more xenon compounds, including the explosive xenon trioxide and its surprisingly well behaved relatives the perxenates, as well as a few krypton compounds and even argon hydrogen fluoride. You can read more about this in THE NOBLE GASES which he wrote after a distinguished teaching career at UBC-2 (University of Berkeley, California). The Wikipedia article on Xenon is bung full of references if you want to chase this further.
However, this all required the intervention of nature’s most voracious element, fluorine, which is also the necessary intermediary in the route to the oxygen containing compounds.
I loved this when I first read about it in the New Scientist in 1962. It was with great interest then, that in 2000 I read an article on compounds of xenon with gold, which the author likened to a match between two noble families. But having only just now searched this out, I find that it is not such an aristocratic match after all. Reading the article Gold-Xenon Complexes from the discoverers themselves, one sees that this again involves the promiscuous fluorine, this time linked also with antimony in a similar way to “magic acid”, so called because it will even protonate paraffins.
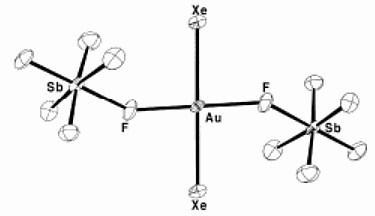
Now, at last comes a fluorine-free xenon compound.
But when I read the paper, I did a double-take. When xenon and hydrogen are forced together under very high pressure in a diamond anvil cell, they form regular crystal-like structures. I had learnt about covalent compounds and ionic compounds, but this was a van der Waals compound. In covalent compounds, electrons are shared between atoms, in ionic compounds one atom gives while another takes. But van der Waals forces are much weaker, no more than a ‘chat over the garden wall’ between one atom and its neighbour. Could this really be described as a compound at all?
 | This beastie is a xenon-hydrogen compound, Xe(H2)7, and pairs of xenon atoms are surrounded by spherical units consisting of freely rotating dumbbell-like hydrogen molecules. |
The crystal structure shown above is at a ‘measly’ 4.95 gigapascals (GPa). How deep in the Earth does that correspond to? Using the simple rule of thumb that near the surface 30 km depth change is close to 1 GPa pressure change, that’s a ‘mere’ 150 km or less than 100 miles down. But taking the core-mantle boundary to be at 120 GPa, and the inner-outer core to be at 330 GPa, these guys get quite close to the inner core with observations up to 255 GPa.
But van der Waals compounds are not unheard-of. Back in 1992, some workers had observed A high-pressure van der Waals compound in solid nitrogen-helium mixtures through their diamond anvil cell. Squashing these two gases together, they not only forced them into a volume less than the two gases separately would occupy at that pressure, but they saw nice angular crystals growing and obtained X-ray diffraction patterns of the crystal lattice. This paper is well worth a read, and the authors argue convincingly that what they are observing is a compound. (“It is, Jim” say I, “but not as we know it”.)
Pressing together these gases may hint at conditions inside giant planets, and in the case of hydrogen, suggest future options for storing this gas when it becomes our main fuel.
** Aficionados of the Earthworm Jim cartoon series will recognize this as the Inert Gases Song which Bob the Goldfish tried to teach to his followers.





Comments