Scientists have just identified several molecules capable of reversing the brain abnormalities of Parkinson’s disease (PD), while also uncovering new clues for its origin in a study just published in the journal Disease Models and Mechanisms (1). PD is characterised by abnormal deposits of a brain protein called alpha-synuclein throughout the damaged brain regions, but exactly what they do there is not clear. The fact that their numbers and spreading are associated disease progression has made them, however, a major point of interest in PD research. The work now published suggests that these deposits are actually a normal physiological process to purge unwanted proteins but, when “overloaded”, they can also cause of the cellular abnormalities seen in PD neurons and, ultimately, neural death. This would explain why the disease tends to appear later in life when the whole metabolism (including this mechanism) becomes less efficient, and also why neurons are particularly susceptible as they are one of the few cells of the body that are not replaced when old and less capable. The study uses a yeast model of PD showing once again the power of simple organism models in the understanding of extremely complex human diseases.
PD is neurodegenerative disease characterised by increasing motor problems - tremors, rigidity and balance and coordination problems - that can leave the patient incapable of perform the simplest of everyday task. Many patients also suffer from non-motor symptoms, including dementia. There is also widespread death of dopamine-producing (dopaminergic) neurons in a part of the brain called the substantia nigra. Since dopamine acts as messenger between this region (the control centre) and other neurons around the body to ensure proper regulation of the body’s movement, these deaths are believed to cause PD motor disability.
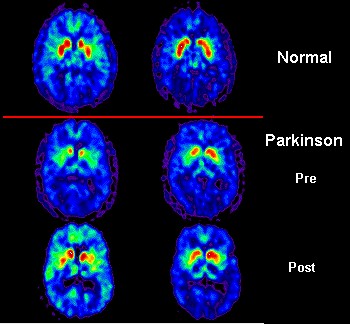
Although the symptoms can be treated with dopamine replacement therapies, as the disease progresses, they stop working and, more importantly, PD is, ultimately, incurable. With the spread of the disease throughout an increasing aging human population bringing dramatic financial and social costs (who will take care of these millions of patients?), new treatments and/or a cure are now being exhaustively researched.
A major focus of the research has been a brain protein of unknown function called alpha-synuclein. In fact, deposits of abnormally folded alpha-synuclein (a certain folding is associated to the proper functioning of each protein) are found in inclusions dispersed all over the damaged brain areas of PD patients. The role of these inclusions in disease is not known with hypotheses ranging from having no importance, to contribute to neural death or even serve to avoid death by rendering harmless toxic misfolded proteins. What is known, however, is that these alpha-synuclein inclusions are excellent markers of disease progression – they accompany the brain degeneration.<?xml:namespace prefix = o ns = "urn:schemas-microsoft-com:office:office" />
In 2003 Tiago Outeiro - a Portuguese scientist and one of the first authors of the new study - and Susan Lindquist – the team leader in both studies – created a yeast model of PD by inserting the alpha-synuclein gene in yeast, an organism that normally does not have the protein. Remarkably, this created in yeast some of the cellular abnormalities seen in PD affected neurons. And as alpha-synuclein quantities increased, also the numbers of inclusions containing the protein, in such a way that led the researchers to suggest that these were, in fact, a physiological process for getting rid of unwanted proteins. And that maybe PD appeared when the capacity of the system was exceed. This hypothesis was supported by the fact that multiplications of the normal alpha-synuclein gene (leading to protein overproduction) were known to cause some forms of human PD, and also by the disease tendency for a late onset, probably due to an aging and less metabolically capable body.
an organism that normally does not have the protein. Remarkably, this created in yeast some of the cellular abnormalities seen in PD affected neurons. And as alpha-synuclein quantities increased, also the numbers of inclusions containing the protein, in such a way that led the researchers to suggest that these were, in fact, a physiological process for getting rid of unwanted proteins. And that maybe PD appeared when the capacity of the system was exceed. This hypothesis was supported by the fact that multiplications of the normal alpha-synuclein gene (leading to protein overproduction) were known to cause some forms of human PD, and also by the disease tendency for a late onset, probably due to an aging and less metabolically capable body.
To test this possibility in the study now published, Linhui Julie Su, Pavan K. Auluck, Tiago Fleming Outeiro and Susan Lindquist, working at the Whitehead Institute for Biomedical Research, Cambridge USA, created yet another yeast PD model this time with even higher levels of alpha-synuclein (High-syn) by inserting extra copies of the gene in the yeast genome. This PD model was then compared with yeast producing none or intermediate levels of alpha-synuclein (Int-syn) (this last organism was the one used in the 2003 study)
Remarkably, the new High-syn yeast suffered from several more of the cellular abnormalities characteristic of PD than the Int-syn yeast. The new abnormalities included problems with mitochondria (the energy producing factories of the cell) as well as accumulation of toxic free radicals, in addition to the abnormalities in lipid transport mechanisms already detected in Int-syn yeast. Problems in mitochondria and accumulation of free radicals are particularly interesting as, although seen in many PD patients, until now they had been impossible to link to alpha-synuclein.
Next, to exploit the fact that the new (High-syn ) yeast PD model shared so many cellular features with its human counterpart the researchers tried to look for possible therapies. For that Su, Auluck and Outeiro tested 115,000 bioactive (so known to affect live cells) compounds and found several able to correct one or more of the cellular abnormalities induced by the high levels of alpha-synuclein. Not only that, but these molecules were also effective treating worm and mammals (rat) models of PD. Even more remarkably, they were capable of rescuing human dopaminergic neurons in a third PD model raising the possibility that they could be used to treat human PD.
Interestingly several of these new potentially therapeutic molecules looked very similar, what led Su and colleagues to test them against each other to find that, in fact, they acted on the same targets across the different species tested. This was particularly important because it shows that the biological mechanisms affected by the over-accumulation of alpha-synuclein are conserved throughout millions of years of evolution - from yeast to humans – further supporting the hypothesis that PD results from a dysfunction of basic cellular mechanisms.
In conclusion Su, Auluck and Outeiro´s work supports the idea that accumulation of alpha-synuclein in vesicles inside brain cells, so typical of PD, is a normal physiological mechanism, most probably to get rid of abnormal proteins. Overload of this mechanism seems enough to cause PD-like symptoms (after all in these yeast models the protein is perfectly normal). Neurons are particularly susceptible not only because they are not renewed throughout the organism’s life, but also because they have higher than normal requirements for both mitochondria and lipid metabolism due to their highly energetic functions.
The new study’s major breakthrough, however, is the identification of several new compounds apparently capable of reversing the cellular abnormalities associated with PD and, as such, with potential to be used in treatments against the brain degeneration of PD.
In fact, at the moment the disease is believed to already affect a striking 3% of the population above 65 years old (more than 1 million in the US, 1,2 millions in Europe) and in a world where life expectancy is steadily increasing, pushing PD numbers (by the age of 80 more than 2 out of 100 people will have signs of the condition), any clues into the disease mechanisms and possible treatments are crucial.
Still, much more work is needed before therapies can be developed “The next step - says Tiago Outeiro - is to confirm these results in other PD models, even more similar to the human disease, to understand better the mechanisms and identify the molecules’ targets so they can, if proven secure, be eventually tested in humans”
1 Disease Models and Mechanims – advanced online publication doi: 10.1242/dmm.004267
“Compounds from an unbiased chemical screen reverse both ER-to-Golgitrafficking defects and mitochondrial dysfunction in Parkinson disease models”






Comments