Okinawa Institute of Science and Technology Graduate University (OIST) reconstructed the 3D structure of one of the proteins of Plasmodium falciparum, the causative agent of malaria and the antibodies that act as the first line of defense against the parasite. This research, published in Cell Reports, was conducted at the Structural Cellular Biology Unit, led by Prof. Ulf Skoglund. This study provides valuable knowledge for the design of anti-malaria drugs.
Plasmodium falciparum is transmitted to humans by the bite of some species of the Anopheles mosquito. Once inside the human body, the parasite soon reaches the liver where it matures and it is then ready to infect red blood cells, also called erythrocytes. The parasite survives by bursting from infected red blood cells and attacking more of them.
One strategy used by the pathogen to amplify its spreading probability is the formation of rosette-shaped clusters of uninfected erythrocytes surrounding a malaria-infected red blood cell. Since the parasite in the central cell of the rosette can easily infect the surrounding cells, the rosette enhances the infection. Moreover, rosetting is associated with severe malaria and high fever. In small blood vessels big rosettes bind to the walls of the capillaries, obstructing the normal blood flow, causing the body to react with high fever. Since children and elderly people have thinner capillaries, they are at higher risk of severe malaria.
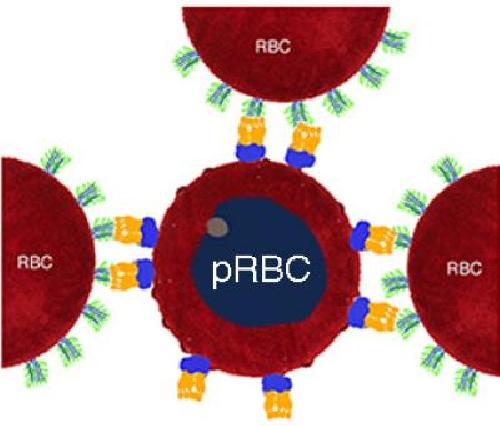
One of the key players in the formation of the rosette is the protein PfEMP1, Plasmodium falciparum erythrocyte membrane protein-1. PfEMP1 sticks out of the infected red blood cell and deceives one of the first defenses against malaria called the IgM antibodies. IgMs bind to the pathogen or pathogen-infected cells and call other immune molecules, like the complement system, for backup. OIST researchers have shown that the IgM bind one or two PfEMP1 proteins, forming a bouquet type shape on the surface of the infected cells. The malaria parasite exploits these IgM to its own advantage, because the bouquet attracts more red blood cells facilitating the formation of rosettes. Moreover, the IgMs in the bouquet are not able to bind the complement system and to destroy the infected cell. "The bond between PfEMP1s and IgMs is like the perfect velcro: not too loose, not too strong. It is devilish engineered to fool our immune system," comments Prof. Skoglund.
The technique used by OIST researchers allows them to have a unique dynamic view of the proteins' conformation. "We have seen that PfEMP1 is a stiff C-shaped protein. Being stiff is an advantage, if it was floppy, it would not work so well. IgM, instead, assume three conformations: extended, bell and turtle shape".
Having this 3D structural model of the PfEMP1 and IgM complex can help scientists to design anti-malaria pharmacological treatments that can break down or wash out malaria rosettes without hurting the patient.





Comments