The historical fact is that Sinsteden's invention was improved by Planté in 1860. The improvement was such as to turn a piece of laboratory apparatus into a commercially viable product. There was already a need for such a product: the electric car, train and boat had already been invented, albeit as models or primitive prototypes.

Wilhelm Josef Sinsteden
In 1748 Benjamin Franklin invented the electrical storage battery by joining a number of Leyden jars in series. The name 'battery' was in regular use by the time of Volta's experiments. When Volta wrote of his invention he called it a battery - it was later writers who adopted the term 'pile', perhaps to distinguish between Volta's two inventions: the 'dry battery' or pile and the 'wet battery' or 'crown of cups'. After Volta published, many investigators sought to explain the cause or causes of this new electrical effect. Those investigations led Sinsteden to the discovery of lead-acid electrochemistry and the invention of the first lead-acid battery, an invention for which far too often Planté is given credit.
It is very strange that so many authors who cite Planté's Recherches sur l'électricité seem not to have actually read it. Planté gives credit to Sinsteden, after discussing battery developments since Volta. He also credits Offershaus and Hare with being first to use rolled coil electrodes. The following is from chapter 1 of an English translation of Planté's Recherches sur l'électricité.
1. Secondary Currents were observed at the beginning of this century, shortly after the discovery of the Voltaic Battery.It is important to note that most experimenters of the period used thin wire electrodes in electrolysis experiments rather like Planté's figure 1 which he describes as a two or four wire voltameter. Sinsteden used, not wires but plates of lead.
Gautherot, a French Scientist, was the first to discover, in 1801, that platinum or silver wires which had been used to decompose saline water by this battery, possessed the property, after having been cut off from the battery itself, of giving an electric current of short duration.
Ritter made the same discovery at Iena, with gold wire, and made the first secondary battery, by superposing a series of pieces of gold, separated by cloth discs, moistened with a saline solution. This battery, inactive in itself, after having been submitted to the action of a voltaic battery of a greater number of cells than that of which it was composed, could give for some moments a current in the opposite direction to that of the voltaic battery. This current took the name of secondary current.
Ritter varied the kind of metal, and the number and surface of the plates composing the secondary battery.
He used platinum, copper, brass, iron, and bismuth, and found that gold, platinum, and silver, gave a stronger secondary current than any of the other metals. He noticed that carburet of iron, and peroxide of manganese, gave still more marked results; but he obtained no effect with lead, tin, and zinc.(i)
(i) We shall explain further on (aa) why Ritter obtained no result with lead, the metal which we have on the contrary exclusively used for obtaining powerful Secondary Currents.
The Secondary Batteries that Ritter employed more especially in his experiments, were formed of discs of copper separated by circular pieces of cloth, moistened in saline water, or sal amoniac. By charging a secondary battery formed of a column of fifty copper discs, by means of a zinc and copper voltaic battery, of a hundred pairs, he obtained decomposition of water, various chemical or physiological actions, and, in general, all the effects produced by ordinary batteries. Nevertheless Ritter's Secondary Batteries, in consequence of the objections arising from their arrangement in a columnar form, or crown of cups, like that given to the voltaic battery itself at that period, in consequence also of the brief duration of the currents produced, and the necessity of employing a battery of a greater number of elements to charge them, could not be advantageously applied either in scientific research or commerce.
2. The secondary current produced by one of Ritter's batteries has been explained by Volta, Marianini, and Becquerel, who have shown that this current arose from the formation of acid and basic deposits upon the metal discs, in consequence of decomposition of the salt impregnating the moistened pieces of cloths, under the influence of the primary current. Becquerel in particular proved clearly the production of an electric current by the reciprocal action of an acid and its base, by surrounding two platinum plates, one with an acid, the other with a basic solution, united by a moist conducting material. He thus formed a battery element of which the plate immersed in the acid solution, constituted the positive pole, and the plate in the basic solution formed the negative pole. The direction of the current of the secondary battery became thus explained, this direction being such that the disc in connection with the positive pole of the primary battery, is itself the positive pole of the secondary battery.
3. About 1826, attention was again called by de la Rive to the secondary current arising from platinum plates in a voltameter filled, not with a salt solution, but with water slightly acidulated by sulphuric acid, or even with distilled water.
As the presence of acid or basic deposits was out of the question in this case, the secondary current was at first attributed simply to physical effect produced by the primary current, to a special polarization of the plates under its influence, and this current received the name of Polarisation Current,
4. This term has remained in scientific use ; but it has since been found that the gases developed around the plates, even in a very small quantity, must be the cause of the current; for Matteucci obtained a current with platinum plates, previously immersed in oxygen and hydrogen gas, and Mr. Grove made a gas battery, by coupling up a certain number of elements formed of platinum plates, immersed at one and the same time in acidulated water, and inverted test tubes of oxygen and hydrogen. This battery was not of course very strong, nevertheless decomposition of water could be obtained by it, and consequently it presented an interesting voltaic circle, the synthesis and analysis of water being exhibited at the same time, in one experiment: the synthesis producing the voltaic current, shown by the gradual absorption of the gas in the test tubes; the analysis produced by the passage of the current in the exterior circuit, demonstrated by the liberation of oxygen and hydrogen gas in a voltameter.
5. Voltaic Polarization has since been the object of interesting labours on the part of a great number of scientists, notably Faraday, Wheatstone, Schoenbein, Poggendorff, Buff, von Beetz, Svanberg, Lenz and Saweljew, Edmund Becquerel, du Moncel, Gaugain, &c.
The object of these works has been, in general, studying or measuring the secondary current obtained with platinum electrodes.
We remember, however, that Mr. Sinsteden,(i) in a memoire upon the results obtained with a magneto-electric apparatus, having by haphasard sent the current from this apparatus through voltameters with lead, silver, and nickel plates, obtained secondary currents with these metals, sufficiently intense to raise wires to a state of incandescence.
(i) Sinsteden. Recherches sur le degre de force et de continuity du courant d'un grand appareil magneto-electrique de rotation. Annals de Poggendorff. t. LXXXXII, p. 16, 1854.
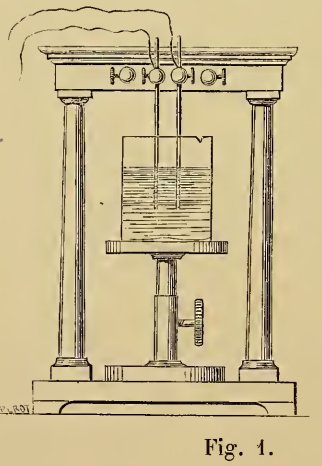
Planté's voltameter, figure 1
In 1854, Sinsteden published his findings on the use of flat lead plates as electrodes and of dilute sulphuric acid as an electrolyte. He discovered that over the course of a number of charge - discharge cycles the storage capacity of his lead-acid cell increased. On investigation he found that the charge cycle formed lead dioxide (lead (IV) oxide) on one plate and pure lead on the other. By increasing the area of the lead plates the output current was increased.
Planté's improvement over Sinsteden's method was in increasing the area of the plates within a compact structure. That improvement made Sinsteden's invention commercially viable.
Planté had investigated the use of various metals in his voltameter and had concluded that the lead / lead (oxide) pair produced the most useful secondary E.M.F.
We have found, ... that the secondary E.M.F. of a voltameter with lead plates in water acidulated with sulphuric acid, was higher and more lasting than that of any of the other metals, and that it surpassed that of the strongest voltaic element known - that of Grove or Bunsen.
... it only remained, in order to form a secondary element of great power, to endow it with a very low resistance, or to increase its surface to the greatest possible extent.
This was so much the more easy from the fact that the two plates necessary to form it were of the same nature and of a metal so extremely flexible and malleable as lead.
It is thus that we were led to construct, in 1860, a secondary element of great power or quantity, by using an arrangement similar to that which Offershaus and Hare had employed for the voltaic cell proper, namely, by rolling two long, wide lead plates into a coil, separated one from the other by a thick cloth, and then immersing them in a glass jar full of water acidulated with a tenth part sulphuric acid.

Figure 7, secondary cell with coiled lead plates.
A short biography of Wilhelm Josef Sinsteden
Wilhelm Josef Sinsteden (May 6 1803 - November 12 1891) was a physician and physicist. He is also known as Josef Sinsteden and by his Latin name: Gulielmus Josephus Sinsteden. He was born in Cleves (Kleve), the fifth of nine children of Michael Franz Severin Sinsteden and Katharina Josepha Sinsteden (Nolten). He was educated both in school and privately. He attended the Dreikönigsgymnasium from 1811 to 1812, after which he left to be taught privately
He went to college in 1815. In 1819 he left high school and went to Cleves. In 1823 he enrolled at the Friedrich-Wilhelm Institute to study medicine and surgery. From 1827 he served as a junior surgeon at the Royal Charité Hospital (now the Campus Charité Mitte).
In 1828 he joined the army as a company surgeon ( Compagniechirurg ) having gained a doctorate of the University of Berlin for his Diss. sistens rationem gravitatem inter vim et vital .
In 1836 he became a staff surgeon and in 1839 a regimental physician in Pasewalk, serving as such during the First Schleswig War of 1848-51. He left the army in 1871 and went to Xanten in 1878 where he died November 12 1891.
During his life he carried out many experiments in physics and wrote a large number of works in the field of optics and electricity.
In 1854, Sinsteden published his findings on the use of flat lead plates as electrodes and of dilute sulphuric acid as an electrolyte. He discovered that over the course of a number of charge - discharge cycles the storage capacity of his lead-acid cell increased. On investigation he found that the charge cycle formed lead dioxide (lead (IV) oxide) on one plate and pure lead on the other. By increasing the area of the lead plates the output current was increased.
Sinsteden probably originated the term 'windmill effect', although the effect itself had been observed and recorded earlier by Robert Smith in in his book A Compleat System of Opticks (1738) and by Porterfield in 1759. A rotating object seen in silhouette will suddenly appear to change the direction in which it is rotating because a silhouette does not provide the observer with depth cues.
we sometimes Mistake the Course of it's circular Motion" (Porterfield 1759, page 380).
image courtesy J. B. Calvert
A directory of Sinsteden's scientific papers may be found in Poggendorff's Biogr-litterarischem Handwörterbuch II, 939
Sources:
Planté - Recherches sur l'électricité (1883)
Planté - The Storage Of Electrical Energy (1859)
German Wikipedia
http://de.wikipedia.org/wiki/Wilhelm_Josef_Sinsteden
http://de.wikipedia.org/wiki/Bleiakkumulator#Geschichte
Dictionary of German Biography, Walther Killy (Ed.) et al., Vol. 9
Google books: Ratio gravitatem inter et vim vitalem. Author, Wilhelm Joseph Sinsteden. Published, 1828. Original from, the Bavarian State Library. Digitized, Jan 30, 2012
Deutsche Biographie - Sinsteden, Wilhelm Joseph
Strasser Familiengeschichte
Poggendorff's biographical master list is not available on line except in parts. For those who wish to research further, here is the full description:
Poggendorff, J. C. (Johann Christian), 1796-1877.
Biographisch-Literarisches Handwörterbuch zur Geschichte derExakten Wissenschaften: enthaltend Nachweisungen über Lebensverhältnisse undLeistungen von Mathematikern, Astronomen, Physikern, Chemikern,Mineralogen, Geologen usw. aller Völker und Zeiten, gesammelt von J.C. Poggendorff, Mitglied der Akademie der Wissenschaften zu Berlin.
Leipzig, 1863 - [In German.]
.




Comments