It has been developed as a tool for imaging and quantification of cellular and molecular processes in vivo and made an important role in staging diseases and monitoring response to treatment (2).
In comparison to anatomic imaging modalities, PET produces images of biochemical and physiologic processes in tissues that help distinguishing benign and malignant lesions when CT and MRI cannot (1,3) .
The steps involved in PET imaging are the following: selection and production of a suitable molecular probe, pharmaceutical labeled with a positron-emitting radionuclide, administration of the probe to the patient, and imaging of the distribution of the probe in the patient (4).
Detection of coincidence photons emitted during positron annihilation is the key in this modality (5) and can be attained through understanding its basis, that is, the labeling of small, biologically important moleculesa with positron-emitting radionuclides. Thereby, positions of positron-emitting tracers can be detected by the scanner when it undergoes radioactive decay and the “physiologic map” of the functions or process relevant to the labeled molecules can be created by imaging the temporal distribution of these labeled compounds (1).
To discuss the process thoroughly, positron emitters achieve stability through nuclear transmutation of a proton into a neutron, and it involves the emission of a positive electron and an electron neutrino. The positron loses energy through interactions with the surrounding tissue until it annihilates with an electron followed by the emission of 2 annihilation gamma rays in opposite directions, thus are detected in coincidence.
One example of a neutron-deficient isotope is 18Fb which is used to label substratesc to create a radiopharmaceutical Fluorodeoxyglucose (FDG). The radioactive label is then transported to the organ of interest by the circulation and through metabolism of the pharmaceutical it is incorporated into the organ. Radiopharmaceutical is then injected into the patient who is positioned in the PET scanner. Gamma ray pairs from positron annihilation are captured in coincidence by opposing detectors.
These pairs of coincident photons detected are stored in sinograms where each row in the matrix represents a parallel projection of the activity distribution in the patient at a specific angle and axial position. An image reconstruction algorithm is applied to the sinograms to recover the underlying radioactivity distribution, thus indirectly mapping the functional process that created the distribution of positron emitter. The images produce by this isotope are FDG accumulation throughout the body that is closely related to tissue glucose utilization (4).
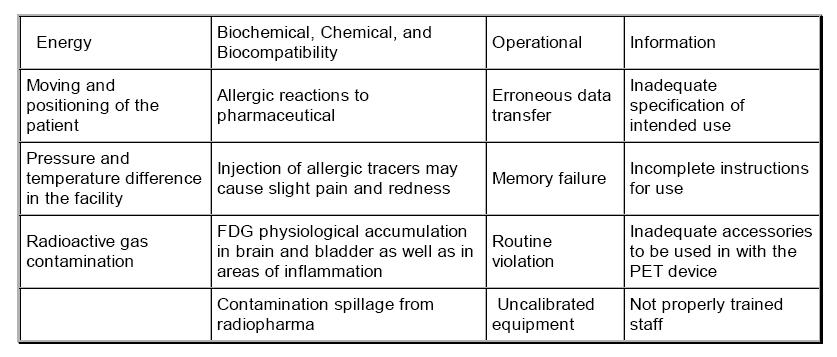
There are many potential benefits of PET, especially in radiation oncology such as indication for non small cell lung cancer, melanoma, lymphoma, colorectal ca, cervical ca, etc. (1). However, potential benefits of PET should be used to offset or justify the risk of another. Like any other modality, it must be used based on evidence because it can pose risk, as well as benefits (6). List of examples of hazards based on ISO 14971:2007(E) that may arise in PET are tabulated below.
Table 1. Examples of hazards of PET
_____________________________________________________________________________
asuch as sugars, amino acids, nucleic acids, receptor-binding ligands, water and molecular oxygen
bthe positron-emitting radioactive isotope fluorine-18 in the Fluorodeoxyglucose molecule (FDG)
clike deoxyglucose
_____________________________________________________________________________
References
1) Griffeth LK. Use of PET/CT scanning in cancer patiens: technical and practical considerations.
BUMC Proceedings 2005;18:321-330.
2) Hendrikse NH, Luurtsema G, AM van der Veldt A, Lubberink M. Positron emission tomography for modeling pathophysiological processes in vivo. Current Opinion in Drug Discovery&Development 2008 11(5):717-725.
3) Yang DJ. Book Review: Basics of PET Imaging: Physics, Chemistry, and Regulations. Journal of
Nuclear Medicine 2006; 47:4727
4) Townsend DW. THEME PAPERS Review Article: Physical Principles and Technology of Clinical
PET Imaging. Annals Academy of Medicine Singapore 2004; 33:133-45.
5) Kapoor V, McCook BM, Torok FS. An Introduction to PET-CT Imaging. RSNA Radiographics
2004;24:523-543.
6) Members of the Ontario PET Steering Committee. PET Scan Primer: A guide to the implementation of Positron Emission Tomography Imaging in Ontario. August 2008.
7) International Standard ISO 14971:2007(E). Medical Devices – Application of risk management to medical devices. Second edition 2007:50-51.
8) Nelson RM. Practical Pediatric PET Imaging. 2006:59-71.
9) Radiology Info. Positron Emission Tomography – Computed Tomography (PET/CT). Reviewed
September 2008.
10) Taylor K, Bahen ME, McFarlane N, Laframboise L, McNeill FE, Boreham DR. The Low Dose
Radiation Risk Associated with Diagnostic PET Scans. Department of Medical Physics and Applied
Radiation Science, McMaster University, Hamilton, Ontario, Canada.
11) Fahey, F. Data Acquisition in PET Imaging. Journal of Nuclear Medicine Technology 2002;30:39-49.
Thanks to Mr. Alvin Eufemio for the actual example of hazards






Comments