Stem cells are generally assigned to one of two categories: embryonic or adult. Some researchers say that may be an oversimplification and a new University of Michigan study states that stem cells in the developing fetus are distinct from both embryonic and adult stem cells.
Researchers note that fetal blood-forming stem cells in umbilical cord blood behave differently than adult blood-forming stem cells after transplantation into patients, which would mean that fetal stem cells comprise a separate class.
A University of Michigan team has identified the first known gene, Sox17, required for the maintenance of blood-forming stem cells in fetal mice, but not in adult mice. The discovery provides a critical insight into the mechanisms that distinguish fetal blood-forming stem cells from their adult counterparts.
The Sox 17 study is part of a larger, ongoing University of Michigan effort to understand how stem cells are regulated at different stages of life. Last September, Sean Morrison's team, which also led this research, reported that old stem cells don't simply wear out; a gene called Ink4a actively shuts them down.
"Each time we identify one of these genes, we get a new insight into what stem cells really are, what regulates their identity and how their age-specific functions work," Morrison said.
Stem cells generate all of the tissues in the developing human body and later in life provide replacement cells when adult tissues are damaged or wear out. Stem cells that form blood and immune-system cells are called hematopoietic stem cells.
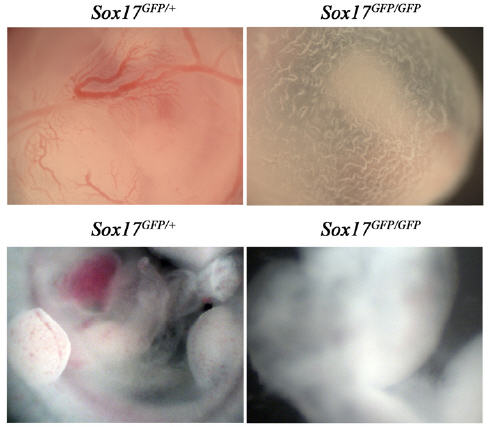
In the latest study, Injune Kim and Morrison looked for genes required to maintain hematopoietic stem cells in fetal mice, but not in adult mice. They found that Sox17 was turned on in fetal and neonatal hematopoietic stem cells but not adult hematopoietic stem cells.
To test whether Sox17 was functionally important for fetal and neonatal blood-forming stem cells, the researchers deleted the Sox17 gene in laboratory mice. This led to the loss of fetal and neonatal, but not adult, hematopoietic stem cells.
In follow-up experiments, mice were irradiated to destroy their blood-forming stem cells. Then replacement fetal or neonatal blood-forming cells were transplanted into the mice---some containing the Sox17 gene and others lacking it. The mice that received Sox17-bearing cells were able to regenerate their blood systems. Those that received cells lacking Sox17 could not.
"Sox17 is really a critical player," Morrison said. "If you knock it out in mice, they never develop a blood system. They never form blood cells."






Comments