Using an innovative device with microscopic chambers, researchers have gleaned important new information about how bacteria survive in hostile environments by forming antibiotic-resistant communities called biofilms.
These biofilms play key roles in cystic fibrosis, urinary tract infections and other illnesses, and the researchers say their findings could help in the development of new treatments and preventive measures.
“There is a perception that single-celled organisms are asocial, but that is misguided,” said Andre Levchenko, assistant professor of biomedical engineering in The Johns Hopkins University’s Whiting School. “When bacteria are under stress—which is the story of their lives—they team up and form this collective called a biofilm. If you look at naturally occurring biofilms, they have very complicated architecture. They are like cities with channels for nutrients to go in and waste to go out.”
With a better understanding of how and why bacteria form biofilms, researchers may be able to disrupt activity in the bacterial communities and block harmful effects on their human hosts. The team’s findings were detailed in an article published in the November 2007 issue of the journal Public Library of Science Biology.
In the article, the researchers from Johns Hopkins; Virginia Tech; the University of California, San Diego; and Lund University in Sweden reported on the observation of the bacteria E. coli growing in the cramped conditions of a new microfluidic device. The device, which allows scientists to use nanoscale volumes of cells in solution, contains a series of tiny chambers of various shapes and sizes that keep the bacteria uniformly suspended in a culture medium.
Levchenko and his colleagues recorded the behavior of single layers of cells using real-time microscopy. Computational models validated their experimental results and could predict the behavior of other bacterial species under similar pressures. “We were surprised to find that cells growing in chambers of all sorts of shapes gradually organized themselves into highly regular structures,” Levchenko said. “The computational model helped explain why this was happening and how it might be used by the cells to increase chances of survival.”
The microfluidic device, which was designed and fabricated in collaboration with Alex Groisman’s laboratory at UCSD, allows the cells to flow freely into and out of the chambers. Test volumes in the chambers were in the nano-liter range, allowing visualization of single E. coli cells. Ann Stevens’ laboratory at Virginia Tech helped to generate new strains of bacteria that permitted visualization of individual cells grown in a single layer.
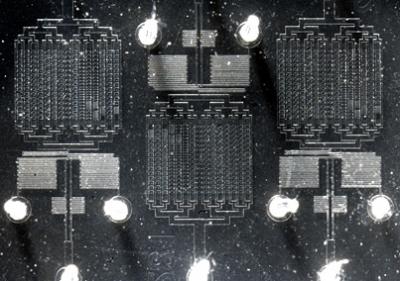
Hojung Cho, a Johns Hopkins biomedical engineering doctoral student from Levchenko’s lab and lead author of the journal article, captured on video the gradual self-organization and eventual construction of bacterial biofilms over a 24-hour period, using real-time microscopy techniques. The experiments were matched to modeling analysis developed in collaboration with Cho’s colleagues at Lund. Images were analyzed using tools developed with the participation of Bruno Jedynak of the Johns Hopkins Center for Imaging Science.
Observation using microscopy revealed that the longer the packed cell population resided in the chambers, the more ordered the biofilm structure became, Levchenko said. Being highly packed in a tiny space can be very challenging for cells, so that any type of a strategy to help colony survival can be very important, he adds.
Levchenko also noted that rod-shaped E. coli that were too short or too long typically either did not organize well or did not avoid “stampede-like” blockages toward the exits. The shape of the confining space also strongly affected the cell organization in a colony, with highly disordered groups of cells found at sharp corners but not in the circular shaped microchambers.
Understanding how bacteria produce biofilms is important to researchers developing better ways to combat the diseases associated with them, Levchenko pointed out. For example, people who suffer from cystic fibrosis—a genetic disorder that affects the mucus lining of the lungs—are susceptible to a species of bacteria that colonizes the lungs. Patients choke on the colony’s byproducts. Chronic urinary tract infections result from bacterial communities that develop inside human cells. And biofilms cause problems in tissues where catheters have been inserted or where sutures have been used.
“You can put a patient on antibiotics, and it may seem that the infection has disappeared. But in a few months, it reappears, and it is usually in an antibiotic-resistant form,” Levchenko says. To explore possible treatments, Levchenko said, the microfluidic device could be used as a tool to rapidly and simultaneously screen different types of drugs for their ability to prevent biofilms.
This research was supported by funding from the National Science Foundation, the National Institutes of Health and the Swedish Research Council.
Citation: Cho H, Jönsson H, Campbell K, Melke P, Williams JW, et al. (2007) Self-Organization in High-Density Bacterial Colonies: Efficient Crowd Control. PLoS Biol 5(11): e302 doi:10.1371/journal.pbio.0050302






Comments