The ozone hole over Antarctica has shrunk 30 percent as compared to last year's record size. According to measurements, this year’s ozone loss peaked at 27.7 million tons, compared to the 2006 record ozone loss of 40 million tons.
Ozone is a protective layer found about 15 miles above us, mostly in the stratospheric stratum of the atmosphere that acts as a sunlight filter shielding life on Earth from harmful ultraviolet rays. Over the last decade the ozone layer has thinned by about 0.3% per year on a global scale, increasing the risk of skin cancer, cataracts and harm to marine life.
The thinning of the ozone is caused by the presence of ozone destructing gases in the atmosphere such as chlorine and bromine, originating from man-made products like chlorofluorocarbons (CFCs), which have still not vanished from the air but are on the decline as they are banned under the Montreal Protocol, which was signed on 16 September 1987.
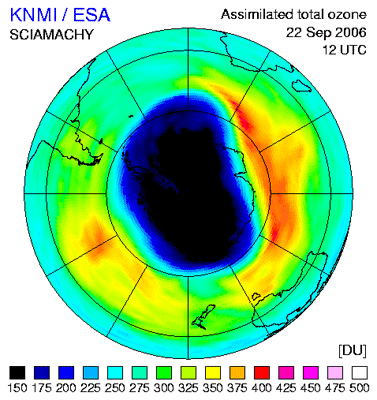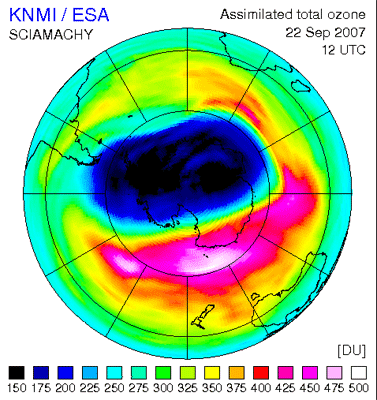
Ozone loss is derived by measuring the area and the depth of the ozone hole. The area of this year’s ozone hole – where the ozone measures less than 220 Dobson Units(1) – is 24.7 million sq km, roughly the size of North America, and the minimum value of the ozone layer is around 120 Dobson Units.
Scientists say this year’s smaller hole – a thinning in the ozone layer over the South Pole – is due to natural variations in temperature and atmospheric dynamics (illustrated in the time series to the right) and is not indicative of a long-term trend.
During the southern hemisphere winter, the atmospheric mass above the Antarctic continent is kept cut off from exchanges with mid-latitude air by prevailing winds known as the polar vortex. This leads to very low temperatures, and in the cold and continuous darkness of this season, polar stratospheric clouds are formed that contain chlorine.
As the polar spring arrives, the combination of returning sunlight and the presence of polar stratospheric clouds leads to splitting of chlorine compounds into highly ozone-reactive radicals that break ozone down into individual oxygen molecules. A single molecule of chlorine has the potential to break down thousands of molecules of ozone.
The ozone hole, first recognised in 1985, typically persists until November or December, when the winds surrounding the South Pole (polar vortex) weaken, and ozone-poor air inside the vortex is mixed with ozone-rich air outside it.
- ESA
(1)A Dobson Unit is a unit of measurement that describes the thickness of the ozone layer in a column directly above the location being measured. For instance, if an ozone column of 300 Dobson Units is compressed to 0º C and 1 atmosphere (the pressure at the Earth’s surface) and spread out evenly over the area, it would form a slab of ozone approximately 3mm thick.






Comments