The results indicate that the normal role for the type of prostaglandin studied, PG F2-alphais to increase blood pressure and accelerate atherosclerosis, at least in rodents, and suggest that targeting this pathway could represent a novel therapeutic approach to cardiovascular disease.
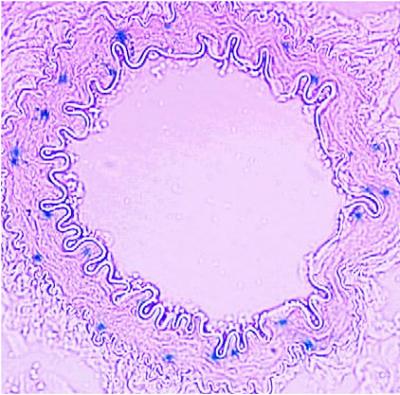
Prostaglandin F2-alpha receptor expression is depicted in blue in a renal artery. Credit: Ying Yu, PhD, and Garret FitzGerald, MD, University of Pennsylvania School of Medicine
"Blocking this prostaglandin receptor may provide a strategy for controlling blood pressure and its attendant vascular disease," notes senior author Garret A. FitzGerald, MD, Director of the Institute for Translational Medicine and Therapeutics at Penn.
To address prostaglandins' role in maintaining blood pressure, FitzGerald and his team, including researchers from the University of Southern Denmark, created strains of mice in which both the maternal and paternal copies of the gene for the PG F2-alphareceptor were deleted. They did this in mice with a normal genetic background and in ones that contained an additional mutation in the low-density lipoprotein receptor gene. These manipulations effectively rendered the mice unable to respond to the prostaglandins.
The delicate balance the body maintains to keep blood pressure stable involves not only the prostaglandin system, but another biological pathway, the renin-angiotensin-aldosterone system, or RAAS. Under conditions of low blood pressure, the liver secretes a protein called angiotensiogen. Renin, an enzyme produced by the kidneys, cleaves angiotensiogen into a peptide called angiotensin I. Angiotensin I is cleaved again to form angiotensin II, which stimulates blood vessels to narrow, thereby increasing blood pressure. At the same time, angiotensin II induces the release of the hormone aldosterone, which further elevates blood pressure by promoting retention of water and sodium in the kidneys.
Many conventional therapies for high blood pressure target components of the RAAS pathway. For instance, ACE inhibitors such as captopril (Capoten) target the formation of angiotensin II, while aliskiren (Tekturna) targets renin.
The team assessed the impact of the PG F2-alpha receptor mutations on both blood pressure and RAAS activity. They found that under a variety of circumstances deletion of the PG F2-alpha receptor lowered blood pressure coincident with suppression of RAAS activity.
"Precisely how these two observations are connected is the focus of our current research," says FitzGerald.
Blood pressure was reduced in both types of genetically engineered mice relative to control littermates. The RAAS molecules renin, angiotensin I, and aldosterone were also reduced, a biological situation leading to lower blood pressure.
The team found that the PG F2-alpha receptor is expressed in the smooth muscle surrounding arteries in the kidneys. However, it was absent in the muscle surrounding the aorta, in the atherosclerotic lesions of mice with their PG F2-alphareceptors knocked out, as well as in the macrophages that inhabit those lesions. Importantly, these atherosclerotic lesions were smaller and less abundant in mice that had both the low-density lipoprotein and PG F2-alpha receptors knocked out, as was macrophage infiltration and inflammatory cytokine production, both of which are indicators of the inflammatory response that marks these plaques.
Prostaglandins are produced during the oxidation of certain cell molecules by cyclooxygenases, the COX enzymes targeted by COX inhibitors, but how remains unclear. FitzGerald's group had previously shown that blockading cyclooxygenase 1 and its major prostaglandin product, thromboxane, also lowers blood pressure, slowing atherosclerosis, but in this previous study, the relevant genes are present in the aorta and its atherosclerotic lesions. PG F2-alpha by contrast, acts via the kidney and represents a distinct therapeutic opportunity.
"The picture is emerging that PG F2-alpha controls blood pressure by a mechanism unique among the prostaglandins," says FitzGerald. "Besides the case of thromboxane, two other types of prostaglandins, PGI2 and PGE2, stimulate renin secretion, which is part of the RAAS pathway."
Assuming these findings can be translated to humans, targeting the PG F2-alpha pathway could represent a novel opportunity for therapeutic control of blood pressure in cardiovascular patients.
The research was funded by the National Heart, Lung, and Blood Institute and the American Heart Association. The results appeared this week in the Proceedings of the National Academy of Sciences.






Comments