The finding in Nano Letters will be most important for small-scale, portable energy applications, where a very compact and lightweight power supply is essential and the fuel supply may be interrupted.
The researchers typically work on thin-film SOFCs that use platinum for the electrodes (the two "poles" known as the anode and the cathode). But when a platinum-anode SOFC runs out of fuel, it can continue to generate power for only about 15 seconds before the electrochemical reaction dies out. The new SOFC uses a bilayer of platinum and VOx for the anode, which allows the cell to continue operating without fuel for up to 14 times as long (3 minutes, 30 seconds, at a current density of 0.2 mA/cm2). This early result is only a "proof of concept," according to principal investigator Shriram Ramanathan, Associate Professor of Materials Science at the Harvard School of Engineering and Applied Sciences, who predicts that future improvements to the composition of the VOx-platinum anode will further extend the cell's lifespan.

Setup for testing solid-oxide fuel cells. The fuel cell is hidden under the circular component at the top, which pins it down to create a tight seal with the hydrogen fuel entering from below. Two needles connect with the electrodes to measure the electricity produced. Credit: Caroline Perry, Harvard SEAS
During normal operation, the amount of power produced by the new device is comparable to that produced by a platinum-anode SOFC. Meanwhile, the special nanostructured VOx layer sets up various chemical reactions that continue after the hydrogen fuel has run out.
"This thin-film SOFC takes advantage of recent advances in low-temperature operation to incorporate a new and more versatile material," says Ramanathan. "Vanadium oxide (VOx) at the anode behaves as a multifunctional material, allowing the fuel cell to both generate and store energy. "There are three reactions that potentially take place within the cell due to this vanadium oxide anode. The first is the oxidation of vanadium ions, which we verified through XPS (X-ray photoelectron spectroscopy). The second is the storage of hydrogen within the VOx crystal lattice, which is gradually released and oxidized at the anode. And the third phenomenon we might see is that the concentration of oxygen ions differs from the anode to the cathode, so we may also have oxygen anions being oxidized, as in a concentration cell."
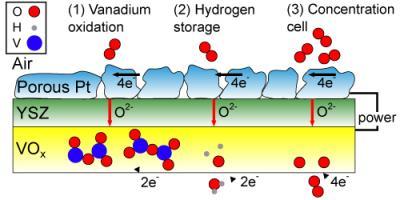
Three possible mechanisms (left to right) can explain the operation of the vanadium oxide / platinum fuel cell after its fuel has been spent. The illustration represents a simplified cross-section of the SOFC: the top layer is the cathode (made of porous platinum), the middle layer is the electrolyte (yttria-stabilized zirconia, YSZ), and the bottom layer is the VOx anode. During normal operation, the hydrogen fuel would be at the bottom of this diagram. Credit: Quentin Van Overmeere, Harvard SEAS
All three of those reactions are capable of feeding electrons into a circuit, but it is currently unclear exactly what allows the new fuel cell to keep running. Ramanathan's team has so far determined experimentally and quantitatively that at least two of three possible mechanisms are simultaneously at work.
They estimate that a more advanced fuel cell of this type, capable of producing power without fuel for a longer period of time, will be available for applications testing (e.g., in micro-air vehicles) within 2 years.






Comments