Stem cell gene therapy has been used to treat a fatal genetic brain disease - Sanfilippo, which in human children causes progressive dementia and death - in mice for the first time.
The researchers are hoping to begin a clinical trial within two years.
Sanfilippo is a currently un-treatable mucopolysaccharide (MPS) disease and affects one in 89,000 children in the United Kingdom alone. Sufferers usually die in their mid-twenties. It is caused by the lack of SGSH enzyme in the body which helps to breakdown and recycle long chain sugars, such as heparan sulphate (HS). Children with the condition build up and store excess HS throughout their body from birth which affects their brain and results in progressive dementia and hyperactivity, followed by losing the ability to walk and swallow.
Dr Brian Bigger, from the University of Manchester's Institute of Human Development who led the research, said bone marrow transplants had been used to correct similar HS storage diseases, such as Hurler syndrome, by transplanting normal cells with the missing enzyme but the technique did not work with Sanfilippo disease. This is because monocytes, a type of white blood cell, from the bone marrow, did not produce enough enzyme to correct the levels in the brain.
Bigger said, "To increase SGSH enzyme from bone marrow transplants, and to target it to the cells that traffic into the brain, we have developed a stem cell gene therapy which overproduces the SGSH enzyme specifically in bone marrow white blood cells. We have shown that mice treated by this method produce five times the normal SGSH enzyme levels in the bone marrow and 11 per cent of normal levels in the brain.
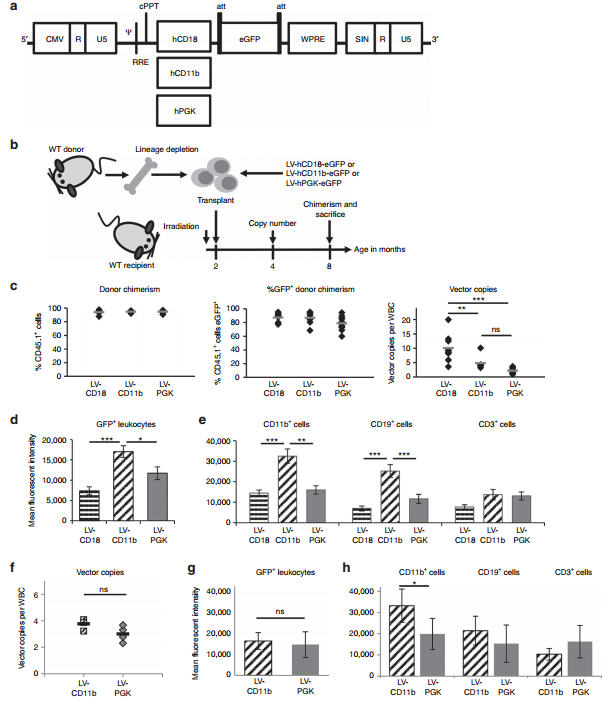
Comparison of eGFP expression from myeloid promoters. (a) pCCL LVs containing human CD18, CD11b, and PGK promoters driving eGFP expression. (b) WT bone marrow was lineage depleted and the enriched stem cell population transduced with LV-hCD18-eGFP, LV-hCD11beGFP, or LV-hPGK-eGFP at an MOI of 40–70 and transplanted into myeloablated WT mice. n= 8–10 (c) Donor and eGFP chimerism was determined 24 weeks post-transplantation. The vector copy number (VCN) was determined in WBCs. (d–e) Mean fluorescent intensity of eGFP expression was determined for all leukocytes and for CD11b, CD19, and CD3 cells 24 weeks post-transplantation. (f–h) Mice with most equivalent copy numbers (VCN of 2.3–4.1) in WBCs were further compared. Error bars represent the SEM. Significant differences between the two groups marked with a line are demonstrated with *P< 0.05; **P< 0.01; and ***P< 0.001. CMV, cytomegalovirus; eGFP, enhanced green fluorescent protein; hPGK, human phosphoglycerate kinase; LV, lentiviral vector; MOI, multiplicity of infection; ns, nonsignificant; WBC, white blood cell; WT, wild-type. Credit and link:
doi:10.1038/mt.2013.141
"The enzyme is taken up by affected brain cells and is enough to correct brain HS storage and neuro inflammation to near normal levels and completely corrects the hyperactive behaviour in mice with Sanfilippo. This is extremely exciting and could have huge implications for treatments. We now hope to work to a clinical trial in Manchester in 2015."
The team is now manufacturing a vector, a tool commonly used by molecular biologists to deliver genetic material into cells, for use in humans and hope to use this in a clinical trial with patients at Central Manchester University Hospital NHS Foundation Trust by 2015.
The stem cell gene therapy approach was recently shown by Italian scientists to improve conditions in patients with a similar genetic disease affecting the brain called metachromatic leukodystrophy, with results published in the journal Science earlier this month.
Manchester scientists refined the vector used by the Italian scientists. "This approach has the potential to treat several neurological genetic diseases," Bigger added.
The research was funded by the UK MPS Society and the Sanfilippo Children's Research Foundation based in Canada.
Citation: Ana Sergijenko, Alexander Langford-Smith, Ai Y Liao, Claire E Pickford, John McDermott,
Gabriel Nowinski, Kia J Langford-Smith, Catherine LR Merry, Simon A Jones, J Edmond Wraith,
Robert F Wynn, Fiona L Wilkinson and Brian W Bigger, 'Myeloid/Microglial Driven Autologous
Hematopoietic Stem Cell Gene Therapy Corrects a Neuronopathic Lysosomal Disease', Molecular Therapy July 16, 2013 doi:10.1038/mt.2013.141






Comments