For life to begin, there had to be a source of organic compounds in the prebiotic environment. We now think that some of the compounds were delivered to the Earth on comets, meteorites and dust particles, but others were synthesized in the atmosphere, hydrosphere and in volcanic conditions.
How do we know? This question brings up the important topic of prebiotic simulations. In a simulation, we make a set of assumptions about local conditions on the early Earth, then reproduce those conditions in the laboratory and run experiments to see what happens.
The results of the experiment either support a plausible scenario related to the origin of life, or rule out an implausible one. Plausibility, of course, is a judgment call on the part of the experimenter, so the interpretation of simulations can lead to controversy. I think at this point we should consider all possible scenarios, because we are exploring a wilderness and no one yet knows which trail will lead to understanding life’s beginning. Each experiment, whether a failure or success, increases our knowledge of the terrain, and as we explore we occasionally discover a major clue.
Fifty years ago, one such discovery transformed origins of life research from speculation to solid experimental science.
In the early 1950s, a young graduate student named Stanley Miller began his PhD research at the University of Chicago. Harold Urey was his research advisor, and Urey had already won a Nobel prize for his discovery of the hydrogen isotope called deuterium. He was also first to isolate deuterium oxide, or “heavy water” abbreviated D2O.
Urey knew that the outer planets were very high in hydrogen content, along with water, methane and ammonia, and reasoned that the Earth would have had a similar atmosphere just after it completed the process of planet formation. Miller decided to simulate these conditions in the laboratory by enclosing a mixture of gases in a large round flask. As a chemist, he knew that nothing would happen unless some form of energy was driving the reactions, so he chose to use an electrical spark to simulate lightning strikes.
The results were spectacular, and even today remain a touchstone for research on the origin of life. Urey himself was skeptical about the experiment and expected the products to be a tarry mess containg thousands of compounds. He was partly right about the thousands, but the unexpected result was that several of the products appeared as purple and blue spots on a paper chromatogram when it was sprayed with ninhydrin dye and heated. The spots were amino acids like glycine, alanine, and aspartic acid. Last year, a reanalysis of some of Miller’s original samples by modern analytical techniques showed that as many as ten different amino acids were present, amounting to half of the twenty amino acids that compose all proteins in life today.
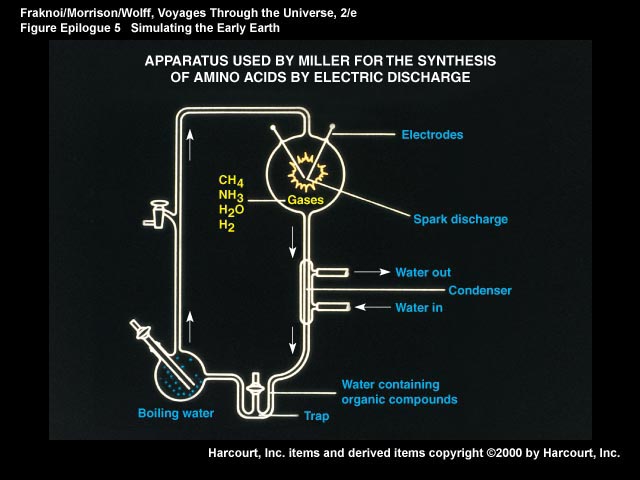
Miller’s paper caused a sensation when it was published in Science in 1953. It demonstrated that the laws of chemistry allow amino acids to be synthesized under prebiotic conditions. After the Miller paper broke the dam, a great deal of effort went into determining how other monomers of life might be synthesized under prebiotic conditions. For instance, nucleic acids are polymers composed of sugars (ribose for RNA and deoxyribose for DNA), phosphate and bases called purines and pyrimidines. Phosphate is a component of certain minerals, so it is a reasonable assumption that small amounts of phosphate were available in the prebiotic environment, but what about sugars and bases?
As far back as 1861, Alexander Butlerow showed that formaldehyde (HCHO) reacted with itself to produce hundreds of different carbohydrates. Butlerow’s observation, now referred to as the formose reaction, was adopted by researchers in the 1960s to explain how carbohydrates might have become components of the prebiotic soup. The fact that hundreds of products are produced is a problem, because ribose, the sugar required to produce RNA, is only a minor component of the mixture. However, it was recently discovered that inclusion of borate in the mix dramatically enhanced the synthesis of ribose. This kind of pleasant surprise is constantly popping up in origins of life research, like the tiny flakes of gold in a prospector’s pan that hint at a mother lode somewhere upstream.
In 1960 another chemical simulation produced a major surprise. John Oro, a talented organic chemist who had recently moved to the University of Houston, was interested in a very simple compound called hydrogen cyanide, or HCN. Why cyanide? This is a horrible toxic gas, right? Well, even though it is toxic to life today, cyanide must have played a major role in the chemistry that led to the origin of life. The reason is that HCN contains a triple bond between the C and the N, and that bond makes it highly reactive. For instance, Miller’s experiment worked because the electric spark produces HCN, which reacts with formaldehyde to form amino acids.
What Oro found is that cyanide can also polymerize with itself according to the following reaction:
5 HCN - > H5C5N5.
The surprise was that H5C5N5 is in fact adenine! This compound is one of the four bases of DNA and RNA, and is also present in all forms of life as ATP, or adenosine triphosphate, which supplies energy for most cellular functions. Once again, a simple chemical reaction led to the synthesis of a primary component of life. Such discoveries gave increasing confidence to the expectation that the origin of life could be understood, and also made it increasingly plausible that life could arise on any planet that has liquid water, an energy source and organic carbon compounds.
This is why it is so exciting that we now have clear evidence that our sister planet Mars once had shallow seas. In the next twenty years, robotic Mars rovers may discover evidence for past life there, or even existing life deep beneath the surface where there could still be a source of heat energy and liquid water.
I do want to insert a measured dose of skepticism at this point. We should not conclude that great heaps of organic material were piled everywhere on the early Earth. What is certain is that small amounts of these compounds were likely to be available, just as they are in certain meteorites. For instance, along with amino acids, traces of adenine have been found in the Murchison meteorite (~1 ppm, or part per million, equivalent to 1 microgram per gram of meteorite) and small amounts of simple sugars and phosphate have also been detected.
The fact that such compounds were synthesized in the asteroids from which carbonaceous meteorites are derived adds weight to the argument that similar reactions could have occurred on the young Earth.
In my next column I will discuss the prebiotic synthesis of long chain acids and alcohols, which are essential components of the membranes required for the origin of cellular life.





Comments