Charting the circuitry of the brain and nervous system just got a lot more fun.
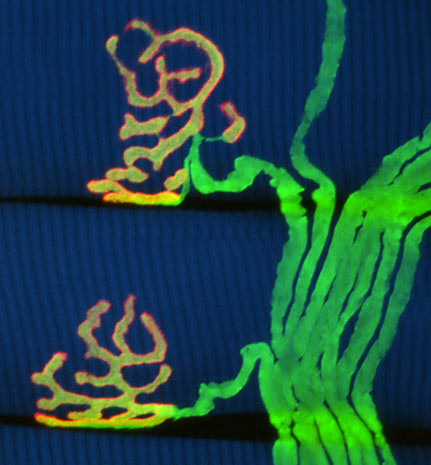
By activating multiple fluorescent proteins in neurons, neuroscientists at Harvard University are imaging the brain and nervous system as never before, rendering cells with 90 distinct colors which they dubbed a "Brainbow."
Brainbow is a huge leap over the handful of shades possible with current fluorescent labeling. By permitting visual resolution of individual brightly colored neurons, this increase should greatly help neuroscientists in understanding how our brains function.
"In the same way that a television monitor mixes red, green, and blue to depict a wide array of colors, the combination of three or more fluorescent proteins in neurons can generate many different hues," says Lichtman, professor in the Department of Molecular and Cellular Biology and the Center for Brain Science in Harvard's Faculty of Arts and Sciences. "There are few tools neuroscientists can use to tease out the wiring diagram of the nervous system; Brainbow should help us much better map out the brain and nervous system's complex tangle of neurons."
Equal parts pointillism, fauvism, and abstract expressionism(!), the resulting images could also help scientists identify how brain wiring goes awry in many different diseases. Brainbow could also help track the complicated development of the mammalian nervous system, currently understood only in general terms. This, in turn, could elucidate the origins of the many brain disorders that arise early in development.
Drawing upon a mix of genetic tricks and special proteins that cause cells to glow, Brainbow uses a well-known genetic recombination system known as Cre/lox in a new way, to shuffle genes encoding green, yellow, orange, and red fluorescent proteins. The researchers painstakingly assembled the Brainbow transgene from snippets of DNA, and inserted it into neuronal DNA. As they predicted, the cut-and-paste recombination occurred totally at random, in the process assigning scores of different colors to neurons. This variation makes neurons leap out from one another visually under ordinary confocal microscopy.
"The technique drives the cell to switch on fluorescent protein genes in neurons more or less at random," says Livet, a postdoctoral researcher in the Department of Molecular and Cellular Biology and Center for Brain Science who did much of the legwork behind Brainbow. "You can think of Brainbow almost like a slot machine in its generation of random outcomes, and Cre/lox is the hand pulling the lever over and over again."
Using Brainbow to look at mouse neural circuits over periods as long as 50 days, the Harvard researchers were able to observe some neural reorganization over time and to ascertain that Brainbow labeling is stable and long-lived. Livet, Sanes, Lichtman, and colleagues are now using Brainbow to scour the nervous system for new insights into its organization and function.
"We've already used Brainbow to take a first peek at the nervous system of mice, and we've observed some very interesting, and previously unrecognized, patterns of neuron arrangement," says Sanes, professor in the Department of Molecular and Cellular Biology and Center for Brain Science at Harvard. "As far as understanding what we're seeing, we've only just scratched the surface."
The technique is described in the cover story of the Nov. 1 issue of the journal Nature by a team led by Harvard's Jean Livet, Joshua R. Sanes, and Jeff W. Lichtman. Co-authors are Tamily A. Weissman, Hyuno Kang, Ryan W. Draft, Ju Lu, and Robyn A. Bennis, all of Harvard's Department of Molecular and Cellular Biology and Center for Brain Science. The work was funded by the James S. McDonnell Foundation and the National Institutes of Health.






Comments