Celiac disease who had persistent intestinal damage - identified with repeat biopsy - showed a higher risk of lymphoma than patients whose intestines healed, according to a new paper.
Celiac disease is an autoimmune disease that affects up to one percent of individuals in Western nations and is characterized by damage to the lining of the small intestine that, over time, reduces the body's ability to absorb components of common foods. The damage is due to a reaction to eating gluten, which is found in wheat, barley, and rye.
When a patient with celiac disease is initially diagnosed, intestinal biopsy shows flattening of villi, the long, fingerlike projections that normally absorb nutrients and fluid. Symptoms of celiac disease, which include diarrhea, weight loss, and iron-deficiency anemia, result from damaged villi. Though not a universal practice, a follow-up biopsy is often done several months to several years after diagnosis, to monitor the effects of dietary changes and treatment on intestinal healing.
"After the diagnosis is made and the patient starts a gluten-free diet, we expect to see recovery of the villi," said Peter Green, MD, professor of medicine and director of the Celiac Disease Center at Columbia University Medical Center, a gastroenterologist at NewYork-Presbyterian/Columbia, and a co-author of the study. "Physicians and patients alike see healing as a goal, but until now there was no confirmed link between healing on intestinal biopsy and clinical risk factors."
"We have known for many years that patients with celiac disease have an increased risk of lymphoma, but we did not know whether intestinal healing and its timing affect that risk," said first author Benjamin Lebwohl, MD, MS, assistant professor of epidemiology at the Mailman School of Public Health and a gastroenterologist at NewYork-Presbyterian/Columbia. "Our study shows that celiac patients with persistent villous atrophy—as seen on follow-up biopsy—have an increased risk of lymphoma, while those with healed intestines have a risk that is significantly lower, approaching that of the general population."
In this study, researchers identified patients with celiac disease who underwent a follow-up biopsy between 6 months and 5 years after their initial diagnosis; the median duration between celiac disease diagnosis and follow-up biopsy was 1.3 years. The patients were then followed for an average of 8.9 years after their follow-up biopsy. Of 7,625 patients with celiac disease, 4,317 (57 percent) had healed on the follow-up biopsy, while the remaining 3,308 patients (43 percent) had persistent villous atrophy.
The investigators found that overall, patients with celiac disease had an annual lymphoma risk of 67.9 per 100,000, a 2.81-fold increase compared with the general population risk of 24.2 per 100,000. Those with persistent villous atrophy had a larger annual risk: 102.4 per 100,000, compared with those with healed intestines, whose risk was 31.5 per 100,000. According to the authors, these findings support the end-point of mucosal healing as a goal for patients with celiac disease so as to reduce lymphoma risk.
A type of blood cancer, lymphoma occurs when white blood cells called lymphocytes, which help protect the body from infection and disease as part of the immune system, divide faster than normal or surpass their typical life expectancy. Lymphoma may develop in the blood or bone marrow, as well as in the lymph nodes, spleen, and other organs; eventually, it may form tumors.
"Guidelines about routine follow-up biopsy are inconclusive, but this study may convince physicians that the follow-up biopsy can carry important prognostic information," said Lebwohl.
One reason this link was not previously seen was a lack of data on the broad spectrum of patients with celiac disease, rather than just those seeking specialized care. Over the past decade, Jonas Ludvigsson, MD, PhD, a pediatrician and professor of clinical epidemiology at the Karolinska Institutet in Stockholm, established a large population-based database using biopsy results from 28 pathology departments in Sweden. "It is only now that we are able to quantify the risk of persistent villous atrophy, which is likely to mirror poor dietary adherence," said Dr. Ludvigsson, senior author of the study.
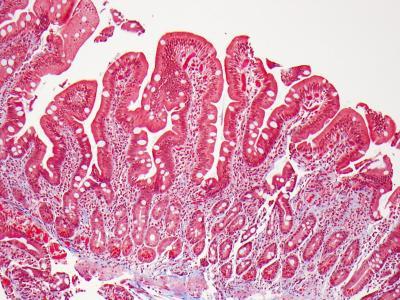
A biopsy of small intestine showing normal villi. Credit: Govind Bhagat, Columbia University Medical Center
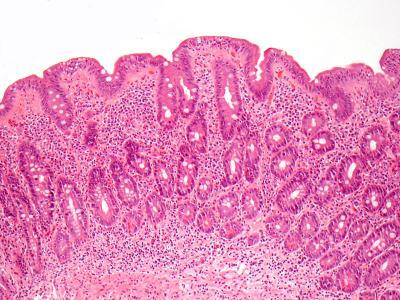
Biopsy of small intestine showing villous atrophy, i.e., flat villi. Image: Govind Bhagat, Columbia University Medical Center.
Why some patients heal while others do not is unclear.
"We know from prior studies that healing is more likely among patients who report strict adherence to the gluten-free diet, compared with those who admit to less-than-strict dietary habits," said Lebwohl.
Since persistent villous damage was seen even in the face of strict dietary adherence, there are likely other, as-yet-unidentified, factors that affect healing.
"Our findings linking the follow-up biopsy result to lymphoma risk will lead us to redouble our efforts to better understand intestinal healing and how to achieve it," said Lebwohl.
Citation: “Mucosal Healing and Risk for Lymphoproliferative Malignancy in Celiac Disease.” The other contributors are Fredrik Granath, PhD, Anders Ekbom, MD, PhD, and Karin E. Smedby, MD, PhD (Karolinska Institutet); Joseph A. Murray, MD (Mayo Clinic); and Alfred I. Neugut, MD, PhD (Columbia University Medical Center/NY-Presbyterian). Upcoming in the Annals of Internal Medicine, no DOI yet.





Comments