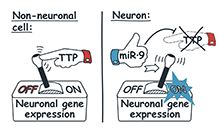
A new study unravels how this synchrony is achieved at the molecular level. The researchers found that many RNA messengers encoding neuronal proteins contain specialized sequences that can promote their destabilization in the presence of an RNA-binding protein called tristetraprolin, or TTP.
This protein is expressed at relatively high levels in proliferating precursors and non-neuronal cells but down-regulated in developing neurons by a brain-enriched regulatory RNA called miR-9. The TTP/miR-9 pair functions as a switch limiting unscheduled accumulation of neuronal messengers in non-neuronal cells and ensuring coordinated accumulation of these molecules in neurons.
“Coherent regulation of multiple genes can pose substantial logistical problems, akin running a successful business employing thousands of people or controlling the vast Galactic Empire from the Star Wars movies,” explains Dr. Eugene Makeyev of Kings College London, a senior author of the study. “Our work suggests that fine-tuning messenger stability is an important mechanism orchestrating gene expression changes during normal brain development.”
Defective regulation of messenger stability, cellular localization and translation into corresponding protein products often leads to serious medical conditions including neurodegenerative diseases and cancer.
A subset of the TTP/miR-9 target genes have been previously linked to these disorders and it will be important to determine whether deregulation of TTP or/and miR-9 plays a causative role in such pathological contexts. Moreover, by uncovering a hitherto unknown mechanism mediating neuronal differentiation, this study should facilitate development of novel cell replacement therapies for neurological and neurodegenerative diseases.
“Natural renewal of neurons in the adult brain is notoriously inefficient and it likely becomes virtually non-existent as we grow older. With a continued increase in average life expectancy, neuron replacement might become a common medical procedure at some point in the future. Luke Skywalker and his aging father would certainly relate to this idea,” said Dr. Makeyev.
The study was supported by grants from the Biotechnology and Biosciences Research Council (BBSRC), National Institutes of Health (NIH) and National Medical Research Council (NMRC).






Comments