Stanford researchers envision a crystal that can form a monolayer three atoms thick. Their computer simulations show that this crystal, molybdenum ditelluride, can act like a switch: its crystal lattice can be mechanically pulled and pushed, back and forth, between two different atomic structures -- one that conducts electricity well, the other that does not.
The switchable material is formed when one atomic layer of molybdenum atoms gets sandwiched between two atomic layers of tellurium atoms. Molybdenum and tellurium are elements that are currently used as additives for making alloys, such as steel. Tellurium is also a component of many modern solar cells.
Such a thin layer might mean a flexible switchable material that could be useful in a world where everyone sits on their smartphones.
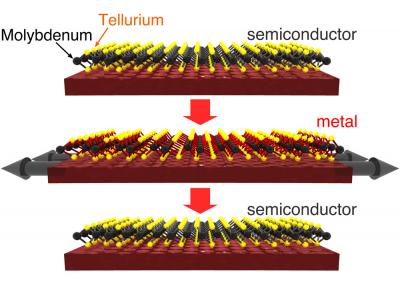
In the top panel, this three-atom thick crystal is shown as semiconductor that is non-conductive. An outward tug on the material (shown in the middle panel) clicks the crystal into a metallic, or conductive state. The third panel shows the crystal back in a non-conductive state. Credit: Karel-Alexander Duerloo
In the simulation, the researchers relied on the fact that molybdenum and tellurium form a sheet-like crystal lattice that is just three-atoms thick. Notably, this atomic sandwich can form different crystalline structures that have useful properties: in one structure this lattice easily conducts electricity; in the other configuration it does not. The simulations show that it takes just a tiny effort to toggle the atomic structure of this three-layer amalgam from a non-conductive state into a conductive state. A gentle push switches the material back to the off state .
These simulations, as yet unsupported by experimental confirmation, are at the leading edge of a new branch of materials science that delves into the behavior of monolayer substances.
The first and most famous monolayer is graphene, which was first observed in 2004. Graphene is a layer of carbon atoms that form a lattice that resembles chicken wire. Although it is just one atom thick, graphene is incredibly strong. A sheet of graphene could bear the weight of a cat without breaking this atomically thin lattice.
Graphene is also electrically conductive. That makes it potentially useful as a light, low power electronic component.
The discoverers of graphene shared a Nobel Prize in 2010, but even before this their work was so honored that other scientists had started looking for other monolayer materials with this interesting confluence of properties: strong, stable, crystalline structures that could conduct electricity.
To help find the most promising materials from a vast universe of molecular structures, a new discipline is rising: computational materials science.
"We're like the advance scouts that survey the terrain and look for the best materials," Reed said.
Now that they have simulated the potential of this molybdenum-tellurium crystal the Stanford researchers – the third team member is graduate student Yao Li -- hope experimental scientists will explore possible uses of this three-atom thick switch.
"No would have known this was possible before because they didn't know where to look," Duerloo said.






Comments