This material originates from volcanoes but in synthesized form it takes up around a third of the average packet of washing powder and it also helps refine 99 per cent of the world's petrol (*) - when it's not used to clean up nuclear waste.
You've probably never heard of it but this extremely useful material is a zeolite. A European team of scientists has revealed, for the first time, its chemical structure using the European Synchrotron Radiation Facility (ESRF). This research opens door to more effective zeolites in the future.
Zeolites are crystalline white minerals, mostly made of aluminium, silicon and oxygen. Their structure is like molecular scaffolding, and thanks to this structure they are frequently used as a “molecular sieve.” This means that with their pores they can separate different molecules and cause different reactions, which are crucial in treating petrol and producing chemicals. Zeolites can also provoke ion exchange, which is useful in water softening or in the removal of nuclear waste (by filtering the radioactive components).
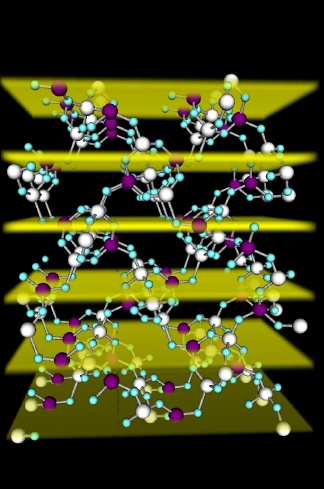
Due to their importance in industry, there is extensive research on zeolites world-wide. However, a crucial aspect about these minerals is still not known. Their functioning and effectiveness depends on different parameters, such as the size of their pores and the distribution of aluminium in the structure of the zeolites. However, the location of the active aluminium remains unknown in many of these materials.
The team from the ETH Zurich, the European Synchrotron Radiation Facility (ESRF), Diamond Light source, the University of Torino and the University of Hamburg have determined unambiguously and directly the distribution of aluminium in zeolites using the technique of X-rays standing wave at the ESRF.
The object of the study was a scolecite zeolite, a natural mineral stemming from the zeolite-rich region of Puna in India. Natural zeolites are not so commonly used in industry because they tend to have more impurities than those synthesised, but they can be used in cleaning nuclear waste. After the Chernobyl catastrophe, tons of zeolites were used with the aim of cleaning the radioactively contaminated area.
The results from the experiments at the ESRF show optimism for the future of zeolites. “By being able to answer the question of where the active sites are, we open up the door to understanding the structure–performance relation. This will lead to ways of improving synthetic zeolites”, explains Jeroen van Bokhoven, corresponding author of the article in Nature Materials.
The next challenge for the team is to study synthetic zeolites with the same technique. Whilst natural zeolites, such as scolecite, contain crystals in the millimetre range, the synthetic ones tend to have much smaller grains, often not larger than a few micrometres. “We have also begun to investigate an industrial synthesised zeolite, but the study is as of yet not complete”, explains Joerg Zegenhagen, in charge of the ESRF beamline where experiments were carried out. “We are currently developing the different beamline elements so that in the very near future we can have the same exhaustive amount of information for synthetic zeolites as for scolecite”, he concludes.
*According to Material World, BBC4, 19 January 2006.
Resources:
The structure of zeolite scolecite, showing the aluminium and silicon atoms as the large sphere, connected by oxygen atoms in blue. The openings in the structure are the pores that serve to transport the molecules through the crystals to the active sites. The planes against which the scattering in the x-ray standing wave experiment occurs are highlighted; the experiment provides the distribution of aluminium and silicon within the crystal, yielding insights into where are the active sites in a zeolite. Credits: J. Van Bokhoven. JPG 245.94k
Article: Van Bokhoven et al., Determining the aluminium occupancy on the active T sites in zeolites using X-ray standing waves, Nature Materials, Advance Online Publication, 22 June 2008.





Comments