This is a rather fun idea by Peter Koch originally suggested in the Moon Miner's Manifest Classics - 1987-1988 (see page 31). It's not so likely in the early stages, because of the large amounts of water needed to construct it, but it may perhaps be of great value at a later stage, especially for bases that have a lot of traffic in and out. If the liquid is water, it has to be over sixty meters deep (62.3 meters), to equalize the pressure inside and outside the habitat. The depth can be much less, if it is a denser fluid. You then don't need any doors but can just dive through it and come out on the surface of the Moon.
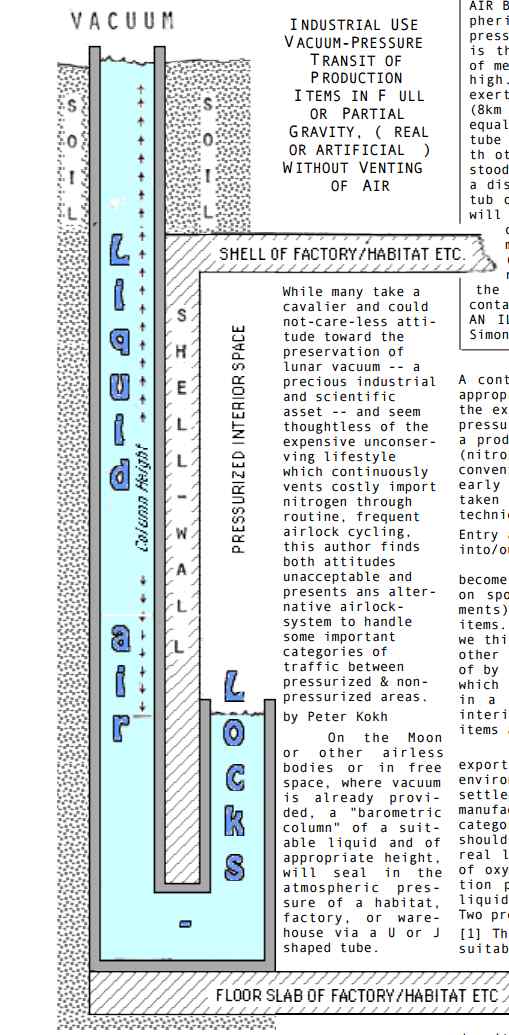
Liquid airlock for the Moon. You need to look carefully at the picture. How it works is that you have a sump, like the sumps that cave divers dive into. On the inside, it is kept in position by the pressure of the air inside the habitat. On the outside is a vacuum.
It's like the way that In a barometer, the weight of the mercury counterbalances the pressure of the atmosphere outside, with a vacuum above the mercury. It's like an "inside out" barometer with the vacuum on the outside. The weight of the water in the column counteracts the pressure of the air in the habitat.
You might think that as for a barometer, mercury is the best fluid of all, because of its high density, at least, if it weren't for its toxicity. Especially for a surface base, the lower column height the better. Mercury would give a column height of only 76 centimeters on Earth, so on the Moon, (9.807/1.622)×0.76 = 4.6 meters. Should we look for the densest liquid we could send to the Moon?
Well, if supplied from Earth, the actual mass of the mercury would be the same as the mass of water. So in that sense, a denser fluid is no saving at all. If you can source the liquid in situ it's also a considerable saving, so if there is ice on the Moon that might well swing it in favour of water rather than some denser liquid from Earth. As for the column height, that's less of a consideration if the base is constructed inside a lunar cave, as many lava tube caves are likely to be at least 60 meters below the surface of the Moon. It might be useful to be able to float up 60 meters to the surface. A denser fluid would help with a surface base.
Peter Koch's motivation is to conserve the air in the base, and also to help preserve the lunar vacuum once we have large scale industry on the Moon,
Whle many take a cavalier and could not-care-less attitude toward the preservation of lunar vacuum -- a precious industrial and scientific asset -- and seem thoughtless of the expensive unconserving lifestyle which continuously vents costly import nitrogen through routine, frequent airlock cycling, this author finds both attitudes unacceptable and presents an alternative airlock- system to handle some important categories of traffic between pressurized & non- pressurized areas.
A continuous loop conveyor provided with the appropriate grip/release system with one end in the external vacuum, the other in the internal pressurized environment, will allow transit on a production basis without the venting of air (nitrogen and/or oxygen) such as occurs in the conventional vestibule-type cycling airlock,
Such a liquid barometric seal could become standard on the Moon (and, for example, on spoke-and-wheel shaped free space settle- ments) to allow entry and exit of routine items. For entry into pressurized environments, we think not so much of imports (from Earth or other settlements) -- these can be taken care of by "match port" docking -- as of those items which it is useful or efficient to manufacture in a vacuum but which will be used in the interior of the settlement. Metal and glass items are possible instances.
For exit, we think not so much of exports of items manufactured in pressurized environments and intended for use within other settlements -- or vehicles -- as of items so manufacture intended for use in vacuum. Of both categories (candidates for entry or exit) there should be several if not many instances. Very real losses of nitrogen, especially, but also of oxygen, can be avoided and vacuum degrada- tion prevented, by the employment of such a liquid airlock system in well chosen cases.
The hard vacuum of the Moon is an asset, it's far better than the vacuum used for chip manufacturing plants on Earth. In early stages then the main contribution towards degrading it is from rocket exhausts, but the retroboosters can be angled during descent so that the high velocity exhaust gas for the most part misses the Moon, and later on, magnetic rail guns and orbital tethers may eliminate that contribution altogether. Geoffrey Landis looked into this in his Air Pollution on the Moon.
It could also be useful as a way to keep the lunar dust out of the habitat if there's frequent cyling of materials from the surface into the habitat.
So, is it practical?
In the vacuum conditions of the luanr surface, exposed water would evaporate quickly. All 62.3 meters of the liquid lock would evaporate away in just one day, or less. But there are solutions to this, such as to use a low vapour pressure liquid, or to replace water with the liauid metal Gallium (which is non toxic, and liquid at 30°C). Another idea (my own suggestion), i sto cover it in a thin film of vacuum stable oil to reduce the evaporation rate.
Some of us have been disucssing this idea on Facebook, in Colonize the Moon, the Space Settlement Alliance, and Case for Moon First, Jocelyn Boily came up with the idea to use a double liquid lock system - the first just uses water, which takes it down to a lower pressure, maybe a tenth or less of the base pressure, and then a final stage uses a material with low vapour pressure such as Gallium, or ionic fluids, or his own favourite, very cold water (to reduce vapour pressure), laced with antifreeze. That way, it doesn't matter if the uppermost layer in the system is denser than water, or dissolves in it or mixes with it. Nick Hoekzema has an intriguing idea about how to use Gallium to simulate full gravity on the Moon - upside down :). I'll update this tomorrrow, 12th October with the new material.
(Mid edit, need to update this with some new ideas from our discussions)
Sections:
- Diving through the waterlock - is that possible in a spacesuit??
- Need for very low vapour pressure liquid - water would lose around 60 meters depth of per day at 0 °C
- Idea to use low viscosity room temperature ionic fluids
- Vacuum stable light oils
- Funicular type railways driving though a sump and out onto the surface
- High density liquids for a lower column - less than 10 meters for gallium
- The high column and low density of water as an asset
- What if the water freezes?
- What about leaks?
- Protection from lunar dust
Diving through the waterlock - is that possible in a spacesuit??
The interesting thing about it is that you could dive through it in a spacesuit, if your suit is capable of being submersed, and come out onto the surface with no loss of air at all from the habitat. There is no need to evacuate the airlock, since it is filled with water rather than air. This means you can have it there available as an airlock all the time, "always open". Any time you want to get out of the base you just dive through the sump. This could be quite a saving in air if the airlock is in regular use and could also make it faster to go in and out - no need to wait for the air inside the airlock to cycle before the next group of people or equipment to exit.
In the other direction it would also keep dust out of the habitat.
Now, spacesuits aren't normally waterproof. The ones used by astronauts to prepare for space missions in swimming pools are just mock-ups of real spacesuits. They are designed to operate in an atmosphere or a vacuum. For one thing, to make the joints mobile, then the bearings lose air constantly at litres an hour, so water would leak in in the other direction. There may also be electronics that would be exposed to water if it was submerged. And the outer layers would not necessarily be waterproof either. Imagine drying off a spacesuit inside a habitat, to stop it getting mouldy?
So - this would need waterproof spacesuits. Perhaps an outer covering that's waterproof and also helps to keep the dust out? Or perhaps you just drive in / out with rovers - in that case the rovers have to be waterproof.
Or, astronauts could have a kind of overall outer suit outside of their spacesuit that they don before getting into the pool, watertight. Even maybe just a kind of big zip-lock bag, zip up get into a lift / hoist, unzip when they get to the top.
For cargo, then deliveries to the ISS are normally packaged anyway - just need to make sure the packaging is waterproof.
Even if not much used for individual astronauts, it could be very useful for large cargo deliveries, if you want to deliver 100 tons at a time, say, then it might take hours to cycle all that material through a small airlock able to take a ton at a time. Either you have large airlocks, or you have a liquid lock. If you do it that way you can just drop the cargo in from the top and remove it from the bottom continuously.
Need for very low vapour pressure liquid - water would lose around 60 meters depth of per day at 0 °C
The liquid needs to have a very low vapour pressure. If it was water you'd lose it through evaporation into space in vacuum conditions at a rate of tens of meters thickness of water per day. Indeed you'd lose nearly all of the 62.3 meters depth of your water lock in a single day of evaporation.
Calculation indented
With surface temperature of 273.15 °K (0 °C) and using the equation for mass loss of liquid water in a vacuum of
(pe/7.2) * sqrt (M/T) kg / m² / sec (equation 3.26 from Modern Vacuum Physics)
where M is the molar mass in kilograms, 0.018 kg for water, T is the temperature in kelvin, pe is the vapour pressure, which for water at 0 °C (273.15 °K) is 611.3 Pa, (Vapour pressure of water at 0 °C), so putting all those into the formula we get:
(611.3/7.2) * sqrt(0.018/273.15) = 0.689 kg / m² / sec.
So you lose 24*60*60*0.689 or about 59.529 tons a day.
So you lose about 60 meters a day thickness of liquid water exposed to a vacuum, or about 21.9 kilometers thickness of water per year at 0 °C.
The rate of loss goes up if the temperature increases. So, what about room temperature? Well they do the calculations here: Modern Vacuum Physics where they use the vapour pressure for water at room temperature 295 K to calculate (2300/7.2) * sqrt(0.018/295) = 2.495 kg / m² / sec.
So at room temperature of 22 °C,you lose 24*60*60*2.495 or about 215.6 tons a day
So now you lose around 215.6 meters per day and 78.6 km per year.
It doesn't help much to have a layer of ice on the surface of the water as ice also sublimes rapidly into water vapour in vacuum conditions, unless it is extremely cold. Spacesuits often use sublimation of ice to cool down astronauts.
So water is no good, so what can one use instead?
Idea to use low viscosity room temperature ionic fluids
The original author suggests NaK. which is highly reactive with water and could burst spontaneously into flame if exposed to air. Not the safest of materials for a liquid airlock
However, this is my suggestion, you could use room temperature or lower ionic fluids. An ionic fluid is a salt, like sodium chloride, common table salt. That's liquid only at very high temperatures. If it is liquid at room temperature or below, you call it an ionic fluid. There, a salt in chemistry is a general term for the result of combining any acid with any base. When in solution, it has positive and negative ions. When you melt the solid salt, if you can do that without it decomposing or vaporizing, it also usually consist of positive and negative ions (cations and anions).
Anyway, so it turns out that if salts have a high molecular weight, they are often liquid at room temperature, and what's more, they can be liquid even at very low temperatures such as you'd get on the lunar surface. That makes them ionic fluids. Typically they have very low vapour pressures, so wouldn't boil away in a vacuum. They have been suggested for liquid mirror telescopes on the Moon.
The only thing is, that most are high viscosity which would make it hard to get in or out, but there is research into low viscosity ionic fluids. Also if the ionic fluid is immiscible and also low density, so that it floats on the surface of the water, you might only need a thin film of it to protect the rest of the water from evaporation. In that case, as a thin surface layer, it wouldn't matter much if it has high viscosity. But many salts will dissolve easily in water.
Vacuum stable light oils
Another idea is to use vacuum stable light oils designed for use in conditions of high vacuum. In this paper, one of the tetraalkasylane oils mentioned has a very low saturated vapour pressure. It's also less dense than water at 857.4 kg / m³ so would float on water. As usual calculation indented so it is easy to skip:
It's structure is:
Figure 1. The structure shown as SiCH-3 has a very low saturated vapor pressure at 25 C of only 2 * 10-10 Torr or about 2.666*10-8 pascals.
So it has
5 * Si: 5*28.0855 amu
4*(3+10) C: 52*12.0107amu
4*(6+21) H : 108*1.00794 amu
For a total of 873.8 amu
That makes the calculation .
(pe/7.2) * sqrt (M/T) kg / m² / sec (equation 3.26 from Modern Vacuum Physics)
where M is the molar mass in kilograms, 0.018 kg for water, T is the temperature in kelvin, pe is the vapour pressure
(2.666*10-8/7.2) * sqrt( 0.8738/(273.15+25)) = 2*10-10 kg / m² / sec. Or about 6.33 g / m² / year .
This time the calculation is for 25 C because that's the temperature at which the author measured the saturated vapour pressure.
An even better example is Pennzane X2000 which is used as a lubricant in space applications
Pennzane X2000 density 0.85, vapor pressure 10-12 torr or 1.333 * 10-10 pascals. This time measured at 40 °C.
Formula C65H13 0 (2 octyldodecyl cyclopentane)
So it's molecular mass is 65*12.0107 + 130*1.00794 amu = 911.7277 amu.
So it would lose (1.333*10-10/7.2) * sqrt( 0.9117277/(273.15+40)) = 10-12 kg / m² / sec. Or about 0.032 g / m² / year .
So, if you cover the liquid water column with a thin layer of this tetraalkasylane oil you'd lose only around 0.26 grams per square meter of the oil to the vacuum of space even if the water was kept at or above normal room temperature at 25 °C. And as for Pentane x2000 then you lose only 0.0013 grams per square meter per year.
You might not need much. Even a micron thick layer, even a monolayer would be enough to reduce the amount of evaporation. A thin layer would also help with the issue of equipment getting covered with oil as it goes in or out of the lock. If it is just a monolayer then this won't have noticeable effects..
You might think that you would need enough thckness to counteract the vapour pressure. For instance, att 0 C then it's equivalent to 44.2 cms thickness of Pennzane as 62.3*0.006033062/0.85 (multipled by the vapour pressure in atmospheres at that temperature, and divided by its density), and and 1.66 meters at 22 C
However, thin layers can help more than expected.Here is a demo someone did as a YouTube video:
This is an early paper on it, From their figure 6, then there is a 30 fold reduction from a 90 microns thick layer of 2 percent eucalyptus reside in parafin oil - that's for 16 mm of Mercury or about 0.02 atmospheres as the "vacuum"
That would be enough to make a significant difference, it's a reduction from 149 kg a minute to 5 kg a minute water loss.
Even a monolayer can make a difference, and also would have more effect in vacuum conditions, they have been found to reduce evaporation of water from reservoirs, up to 40% in experiments in places like Africa
You can also cover with a solid, a thin layer of wax.
"Apiezon® wax W40 is similar to type W, but has a lower softening point which makes it very suitable for flow sealing in or around vacuum joints. It is not recommended for use at temperatures above 30°C. It has a vapor pressure at 20°C of 10^-7 mbar."
These waxes are used for manufacture of integrated circuits. It doesn't say how thick they have to be but a solid probably wouldn't have to be that thick to prevent water molecules evaporating through it.
There is another simpler approach. To have an airtight cover. Water starts evaporating as soon as you lift it - but you only do that momentarily as the cargo or people enter or leave the water, it automatically withdraws but for only a few minutes.
Combine with e.g. a layer that does a 30-fold reductin to 5 kg an hour and f it is just momentarily removed for a minute or two that might be a tolerable loss even with no cover
The oil, or other liquid layer wouldn't need to be photostable - there'd be some sort of a reception area probably covered above the upper entrance to the sump which would protect it from exposure to direct sunlight. Probably also it would be a shelter covered in regolith for protection from solar storms.
Funicular type railways driving though a sump and out onto the surface
Perhaps a liquid airlock might also be useful for moving cargo in and out. Instead of those big hanger like airlocks you get in science fiction movies, maybe you'd just have a truck that drives through a sump filled with ionic fluid - or even - a funicular railway type carriage gets pulled through it? You could have trains that run out of the base onto the lunar surface directly with no need for an airlock so long as they can withstand being submerged. This doesn't seem to be beyond future high tech. The more I think of it, the more possibility it seems to have for future tech.
High density liquids for a lower column - less than 10 meters for gallium
One thing that makes it harder on the Moon is that with a sixth of the gravity, the column is six times higher. A water column would have a height of (9.807/1.622)×10.3 or 62.3 meters which is quite a lot. The author talks about higher density fluids to keep the height difference down. Some ionic fluids are high density so that would help. If we can find a high density low viscosity ionic fluid, that's ideal.
However Wikipedia has a useful section on heavy liquids. Sodium polytungstate is the densest non toxic one I can see there, density 3.01, reducing the column height to 20.7 meters. Clerici solution is denser but toxic.
Gallium is even better at density 6.5 reducing height to 9.6 meters, and if you are using it as a heat sink for the habitat seems like a good choice too. Has to be kept above 30 C, That's a little on the warm side for a swimming pool. However even heavy things would float to the surface easily so that would be a plus. That would reduce the height to less than 10 meters. And - the high temperature wouldn't be much of an issue for cargo or people driving in / out in rovers or buggies. But would have to be very sure it's not going to freeze on you as you travel in / out
As with water, you could cover any of these high density liquids with a thin layer of an immiscible low vapour pressure liquid such as vacuum stable light oil. But now it doesn't matter even if it is rather dense, so long as it is less dense than the liquid it covers. However if we use Gallium it has an extremely low vapour pressure
Back to our forumula:
(pe/7.2) * sqrt (M/T) kg / m² / sec (equation 3.26 from Modern Vacuum Physics)
where M is the molar mass in kilograms, 0.018 kg for water, T is the temperature in kelvin, pe is the vapour pressure
vapor pressure 6.08e-36 pa (from this online calculator) molecular weight 69.723
So it would lose (6.083*10-36/7.2) * sqrt( 0.069723/(273.15+30)) = 1.3*10-38 kg / m² / sec. Or about 4*1O^-28 g / m² / year .
Even after a quadrillion years you still would only lose trillionths of grams per square meter.
You'd need to be heavily weighted to submerge in liquid Gallium, lead weights obligatory. But if you ever get into trouble just drop the weights and up you'd float to the surface.
The high column and low density of water as an asset
There's a plus there, however, for water and for low density ionic fluids, as then the height difference is comparable to the depths below the surface of the floors of the lunar cave entrances. When you dive into the sump at the bottom of the cave, your buoyancy will take you to the surface. That could be especially useful for cargo - a sump that goes all the way from the cave floor to the surface would make it easier to move goods up and down using weights, or floats.
The mass isn't that much for a moderate sized settlement. If you have 60 residents, then that's only one ton per resident, and they would have tons of supplies delivered per resident every year probably.
What if the water freezes?
You might wonder if the area exposed the the lunar surface would freeze. However usually the main issue with a habitat is to keep it cool. The ISS has huge heat rejecting panels. So long as enough heat is supplied from the bottom, the hot water would convect upwards and keep the surface warm. Indeed, it might well be an idea to have some kind of radiator system at the top connected to the sump to use it to convey as much heat as one can upwards from the habitat. After all the surface exposed to a vacuum might as well be in a vacuum flask, the vacuum is a good insulator and it would only lose heat through radiation. It's just a small radiator and wouldn't lose much heat into space.
What about leaks?
First, there is no real risk of the water leaking inwards. It's like a cup upturned and pushed down underwater. The pressure of the air in the habitat keeps the water out. It can only get in if the pressure inside the habitat goes down, at which point the water in the sump would gradually rise. Of course one should have a cover that can go over automatically in case of habitat depressurization to stop the water lock filling the habitat with water.
In the other direction though, it's a more serious failure mode. Suppose something goes wrong with the atmospheric pressure regulation and it increases to 1.1 atmospheres. The waterlock would then require a water height of 68.53 meters. There wouldn't be enough height tin the water column to accommodate this, even if there is water available in a reservoir to automatically top it up. So what do you do?
The failure would be spectacular, if the habitat pressure was self regulating. The sump would immediately start to sink, pushed out by the higher pressure inside the habitat. The habitat air system would respond by pumping in more air to compensate for the increase in volume to be filled. The air would eventually bubble around through the sump and up to the surface. The habitat environment control system would continue compensating for the loss of pressure pumping the air out onto the surface of the Moon.
You could design it though with some play in the system to deal with small pressure variations. First you go down a meter or so to go into the sump, so that a slight increase in pressure won't lead to the water level going so low air bubbles out through the siphon. Then to have a reservoir that automatically fills the sump to raise the water levels if the interior sump level starts to fall,, and enough head room at the top of the sump in the reception area above for the water to rise some meters before it overflows onto the surface of the Moon. And finally then those doors that automatically close if the internal pressure is too high to deal with by just adjusting the column height, or in case of some failure of the regulation system itself.
It would need safety doors to deal with this too. Perhaps just a compartment above the sump, an antechamber, with airproof and waterproof doors that slam shut in this situation.
Protection from lunar dust
A liquid airlock would also help keep out dust. A lot of the dust in the Apollo lunar module came in on the outside of the suits. So it would surely also come in on the outside of vehicles and their wheels, and there'd be a chance of it getting scattered in through an open airlock too from the outside. None of this would happen with a liquid airlock.
WATER LOCK
This is an idea from Bryce Johnson to use water inside the airlock on Mars as a way to protect from the dust and contaminants in the dust such as perchlorates. It could be adapted to the Moon as well. He explained the idea to me in a conversation in the Case for Moon facebook group:
"Crews come in from outside. Hatch is sealed. Air is introduced followed by water that not only neutralizes (or at least sequesters) contaminants but also begins the process of cleaning the suits. As a plus, the air is efficiently forced out of the airlock as the water rises. At no point is the water ever exposed to Martian vacuum.
"Evacuating the water only requires opening a valve to the pressurized inside of the shelter to let the air in. Opening a second valve drains the water out like in a bathtub. The contaminated water goes into a holding tank where solids are filtered out. Depending on the exact nature of contaminants, the water could be processed further to be reused. This system would NOT be part of the environmental system and the water is not intended for human/animal/plant consumption."
It has many of the advantages of a liquid airlock, but as the water is never exposed to the vacuum it can just use ordinary water.
Another suggestion (my own, on reading his idea) - in a larger habitat, with enough space for it, you could also have the hybrid idea of an ordinary airlock leading to a sump that astronauts go through to go into the inner habitat. Again the advantage is that you can use ordinary water as it is never exposed to vacuum. It might permit faster throughput of traffic as a hybrid between the water lock and the liquid airlock. It also provides an extra layer of protection if the outer airlock fails, especially if there is enough water to fill the airlock and seal it after a breach, with ice forming as the water cools down exposed to vacuum conditions.
This is one of the sections of my Case for Moon online and kindle book:
- Case For Moon First: Gateway to Entire Solar System - Open Ended Exploration, Planetary Protection at its Heart - and on kindle - which as the name suggests explores the Case for going to the Moon first in detail, as its main focus.
My other books on related topics are:
- MOON FIRST Why Humans on Mars Right Now Are Bad for Science - and on kindle.
This includes my An astronaut gardener on the Moon and continues some of the themes of the Case for Moon First with a special focus on lunar gardening.
Touch Mars? Europa? Enceladus? Or a tale of Missteps? (equivalent to 1938 printed pages in a single web page, takes a while to load).
You can also buy it on Amazon kindle.
My books are all designed for reading on a computer with links to click to go to the sources, and I have no plans to attempt printed versions of them.
(skip to contents)
FACEBOOK GROUPS
- Touch Mars? Europa? Enceladus? Or a Tale of Missteps?
- Case for Moon for Humans - Open Ended with Planetary Protection at its Core
- Humans to Jupiter's Callisto, Saturn's Titan and Beyond
NOTIFICATIONS OF FUTURE POSTS
You can get notifications of new posts on my Science 2.0 blog by following the announcements on twitter. You can also 'like' my Facebook page: Science20 Blog Alerts
THOUGHTS OR COMMENTS
Any thoughts or comments - do say below. Also if you spot any errors in this, however small, be sure to say.





Comments