There is concern that these fossil sources will be running out in the not too distant future. If we are going to continue to use plastic bottles and synthetic fibres, it would be nice to find polymers from sustainable sources that mimic the ones we use so much now. For PET, help may now be at hand, in the form of poly(dihydroferulic acid).
PET has two distinct modes of employment in everyday life. One is as glassy PET, which is formed by taking it in the molten state at around 280°C and quenching it very quickly to near room temperature. If the cooling is fast enough, it cannot crystallize, and so the liquid hardens just like cooling glass or the polystyrene which is used in window envelopes. One gets a stiff, see-through, plastic film.
On the other hand, if one cools it a bit more slowly, masses of crystals form (the Macrogalleria explains how this happens.) The crystals give the material more strength (suitable for some engineering applications) but because, on a microscopic scale, the higher-density crystalline regions are mixed in with less-dense not-crystalline regions, photons trying to get through tend to “stub their toe”, so to speak, and the scattering of light makes the material rather milky-white and semi-opaque in appearance. This crystalline strength of PET is, most notably, put to good use in polyester fibres.
Now there's a new kid on the block, PTHA or poly(dihydroferulic acid). But how does it square up to PET in these respects? When cooled quickly from the melt, it forms a glass which, on heating, softens at a similar or slightly higher temperature than PET. It does, even, melt at a slightly lower temperature, and can crystallize in a similar manner, so it shows potential as a fibre-forming material but with slightly lower processing costs.
 So how is it made? First, we start with vanillin, source of the well-loved flavour. Vanillin doesn’t come only from vanilla pods, but is produced in large quantity synthetically, one source being that it comprises 5% of lignin, from (paper) pulp waste.
So how is it made? First, we start with vanillin, source of the well-loved flavour. Vanillin doesn’t come only from vanilla pods, but is produced in large quantity synthetically, one source being that it comprises 5% of lignin, from (paper) pulp waste. So, as Mrs Beeton might say, “first catch your vanillin”, then subject it to the following scheme.
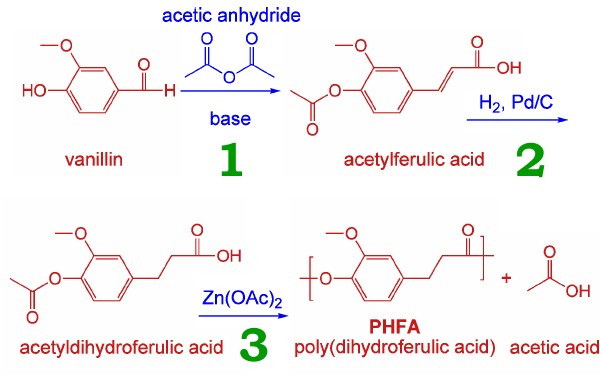
Stage 1 is the Perkin reaction, discovered in the 1860s by that famous chemist (I like good old-fashioned chemistry). Stage 2 is a bog-standard catalytic hydrogenation. One advantage of step 3 over PET production is that it is a single-monomer process, and so cannot get out of balance as can happen with two-monomer processes.
Now how sustainable is this process? First, lignin. It would probably require a major crash of civilization to render this material unavailable. Coming to vanillin, Wikipedia http://en.wikipedia.org/wiki/Vanillin tells us:
By 1981, a single pulp and paper mill in Ontario supplied 60% of the world market for synthetic vanillin. However, subsequent developments in the wood pulp industry have made its lignin wastes less attractive as a raw material for vanillin synthesis. While some vanillin is still made from lignin wastes, most synthetic vanillin is today synthesized in a two-step process from the petrochemical precursors guaiacol and glyoxylic acid.
But with petrochemical processes under pressure, the pendulum may swing back to lignin. Acetic acid and its anhydride, of course, are among the easiest products to obtain from natural sources.
Stage 2 seems to me to be the least sustainable. Since nearly all the world’s palladium comes from either Russia or South Africa, it could also be considered politically vulnerable in the long term.
Stage 3 is not so bad. I do not see zinc becoming critically scarce in the near future.
There will be quite a way to go before this goes commercial. The material has to be put through its paces as regards large-scale synthesis and production of articles, as well as toxicity testing if it is to be used for plastic bottles. But this is one of many scientific efforts which need to be made if we are to continue with civilization as we know it.
Reference to article:
Biorenewable polyethylene terephthalate mimics derived from lignin and acetic acid
Laurent Mialon, Alexander G. Pemba and Stephen A. Miller
DOI:
10.1039/C0GC00150Chttp://pubs.rsc.org/en/Content/ArticleLanding/2010/GC/c0gc00150c





Comments