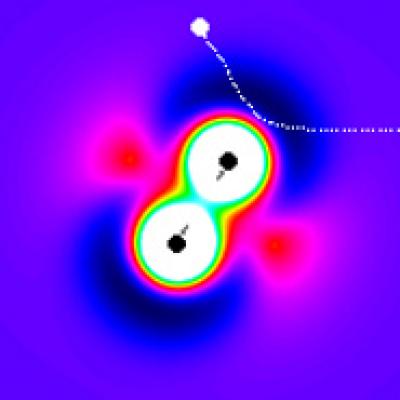
We all know what happens when cars collide on the freeway or an anvil lands on Wile E. Coyote's head - physics at the macro level is predictable. But what about a single hydrogen atom and a lone molecule of deuterium, the smallest atom and one of the smallest molecules?
When an atom collides with a molecule, traditional wisdom said the atom had to strike one end of the molecule hard to deliver energy to it. People thought a glancing blow from an atom would be useless in terms of energy transfer, but that turns out not to be the case, according to the researchers.
Every atom or molecule, even if it has no charge, has electrostatic forces around it-sort of like the magnetic field of the Earth. Those chemical forces exert a pull on any other atom or molecule within range, trying to form a chemical bond.