Research has established that caffeine interferes with processes in cancer cells that control DNA repair, a finding that has generated interest in using the stimulant as a chemotherapy treatment, but given the toxic nature of caffeine at high doses, researchers from the University of Alberta have instead taken advantage of caffeine's lethal effects on cancer cells identify genes and pathways responsible for DNA repair.
The research team found that fruit flies with a mutant gene called melanoma antigen gene, or MAGE, appeared normal when fed a regular diet but died when fed food supplemented with caffeine. On closer inspection, they found the mutant flies' cells were super-sensitive to caffeine, with the drug triggering "cell suicide" - apoptosis.
Through this work, researchers identified three genes responsible for a multi-protein complex, called SMC5/SMC6/MAGE, which regulates DNA repair and the control of cell division. Neither process works properly in cancer cells. The SMC5/6 protein complex consists of the Smc5, Smc6 and Non-Smc-Element (Nse) proteins and is important for genome stability in many species. MAGE (melanoma antigen gene) genes consist of over 50 members, classified into two types; Type I MAGE genes are frequently over-expressed in human primary cancers and cancer cell lines, and may play a role in resistance to chemotherapeutic agents - 85% of cancer cell lines over-express at least one Type I MAGE gene. Type II MAGE genes, such as NDN, MAGEL2 and MAGED1 are expressed in normal tissues and have important roles in mammalian development.
Smc5 mutants are also caffeine-sensitive and Mage physically interacts with Drosophila homologs of Nse proteins suggests that the structure of the Smc5/6 complex is conserved in Drosophila. Flies carrying mutations in Smc5, Smc6 and MAGE were hypersensitive to genotoxic agents such as ionizing radiation, camptothecin, hydroxyurea and MMS, consistent with the Smc5/6 complex serving a conserved role in genome stability.
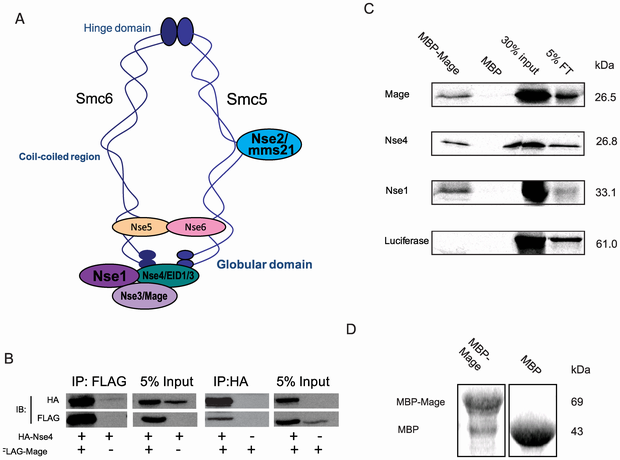
Mage is part of the Drosophila Smc5/6 complex. (A) Diagram of a generic Smc5/6 complex in S. pombe (adapted from [70]). The structure in S. cerevisiae is different in that Nse5/6 were found to bind at the hinge. (B) Mage interacts with Nse4 when both proteins are co-expressed in S2 cells. HA-Nse4 co-immunoprecipitated (co-IP) with FLAG-Mage from an S2 cell lysate when two proteins were co-expressed; FLAG-Mage co-IPed with HA-Nse4 from the S2 cell lysate when two proteins were co-expressed. (C) Recombinant Mage interacts with Nse4 and Nse1 directly. Immobilized maltose binding protein (MBP)-fused MAGE or MBP were incubated with 35S-methionine labeled Mage, Nse4, Nse1, or luciferase (as a negative control), respectively. Proteins that were associated with immobilized MBP-Mage or MBP were resolved with SDS-PAGE and visualized by autoradiography. Results show that Mage, Nse4, and Nse1 each interact with MBP-Mage but not with MBP and luciferase does not interact with either of these proteins. (D) Coomassie staining of protein immobilized on 10 µl of amylose beads showed that approximately equal amounts of MBP-Mage and MBP proteins were immobilized on resin beads. Credit: doi:10.1371/journal.pone.0059866.g003
Publishing their paper in the open access journal PLoS ONE, they write that the finding is significant because it means that scientists one day could be able to take advantage of cancer-cell sensitivity to caffeine by developing targeted treatments for cancers with specific genetic changes.
"The problem in using caffeine directly is that the levels you would need to completely inhibit the pathway involved in this DNA repair process would kill you," said Shelagh Campbell, co-principal investigator. "We've come at it from a different angle to find ways to take advantage of this caffeine sensitivity."
Citation: Li X, Zhuo R, Tiong S, Di Cara F, King-Jones K, et al. (2013) The Smc5/Smc6/MAGE Complex Confers Resistance to Caffeine and Genotoxic Stress in Drosophila melanogaster. PLoS ONE 8(3): e59866. doi:10.1371/journal.pone.0059866





Comments