By simply manipulating chemical gradients in a beaker of fluid, researchers have been able to create delicate flower structures - not at the scale of inches, but microns.
These minuscule sculptures don't resemble the cubic or jagged forms normally associated with crystals, though that's what they are. Rather, fields of carnations and marigolds seem to bloom from the surface of a submerged glass slide, assembling themselves a molecule at a time.
The precipitation of the crystals depends on a reaction of compounds that are diffusing through a liquid solution. The crystals grow toward or away from certain chemical gradients as the pH of the reaction shifts back and forth. The conditions of the reaction dictate whether the structure resembles broad, radiating leaves, a thin stem, or a rosette of petals.
It is not unusual for chemical gradients to influence growth in nature; for example, delicately curved marine shells form from calcium carbonate under water, and gradients of signaling molecules in a human embryo help set up the plan for the body. Similarly, Harvard biologist Howard Berg has shown that bacteria living in colonies can sense and react to plumes of chemicals from one another, which causes them to grow, as a colony, into intricate geometric patterns.
A crystalline tulip, sculpted by chemistry. Credit: Wim L. Noorduin, Harvard University
Replicating this type of effect in the laboratory was a matter of identifying a suitable chemical reaction and testing, again and again, how variables like the pH, temperature, and exposure to air might affect the nanoscale structures.
"For at least 200 years, people have been intrigued by how complex shapes could have evolved in nature. This work helps to demonstrate what's possible just through environmental, chemical changes," says Wim L. Noorduin, a postdoctoral fellow at the Harvard School of Engineering and Applied Sciences (SEAS) and lead author of the paper in Science.

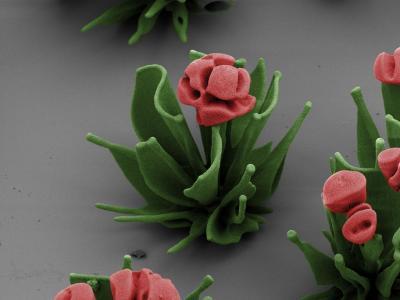
These false-color SEM images reveal microscopic flower structures created by manipulating a chemical gradient to control crystalline self-assembly. Credit: Wim L. Noorduin, Harvard University
To create the flower structures, the researchers dissolved barium chloride (a salt) and sodium silicate (waterglass) into a beaker of water. Carbon dioxide from air naturally dissolves in the water, setting off a reaction which precipitates barium carbonate crystals. As a byproduct, it also lowers the pH of the solution immediately surrounding the crystals, which then triggers a reaction with the dissolved waterglass. This second reaction adds a layer of silica to the growing structures, uses up the acid from the solution, and allows the formation of barium carbonate crystals to continue.
"You can really collaborate with the self-assembly process," says Noorduin. "The precipitation happens spontaneously, but if you want to change something then you can just manipulate the conditions of the reaction and sculpt the forms while they're growing."
Increasing the concentration of carbon dioxide, for instance, helps to create 'broad-leafed' structures. Reversing the pH gradient at the right moment can create curved, ruffled structures.
They have grown the crystals on glass slides and metal blades; they've even grown a field of flowers in front of President Lincoln's seat on a one-cent coin.
"When you look through the electron microscope, it really feels a bit like you're diving in the ocean, seeing huge fields of coral and sponges," describes Noorduin. "Sometimes I forget to take images because it's so nice to explore."






Comments