One hypothesis says microbes that shed necessary functions are getting others to 'do that work' for them, an adaptation that can encourage microorganisms to live in cooperative communities. Yes, genetic inter-organism cooperation and adaptive gene loss.
Richard Lenski and J. Jeffrey Morris of Michigan State University, and Erik Zinser of the University of Tennessee call it The Black Queen Hypothesis - named for the queen of spades in the game Hearts, in which the usual strategy is to avoid taking that card - and it posits that some of the needs of microorganisms can be met by other organisms, enabling microbes that rely on one another to live more efficiently by paring down the genes they have to carry around. In these cases, they say it would make evolutionary sense for a microbe to lose a burdensome gene for a function it doesn't have to perform for itself.
As an illustration of the hypothesis, the authors apply it to one particular microbial system that has been a source of some confusion: one of the most common plankton species in the open ocean, Prochlorococcus, which has a much smaller genome than you might expect. Scientists have wondered how Prochlorococcus has managed to be extremely successful while shedding important genes, including the gene for catalase-peroxidase, which allows it to neutralize hydrogen peroxide, a compound that can damage or even kill cells.
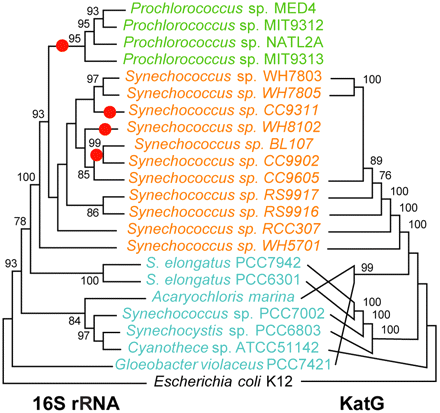
Comparison between the phylogenies of the catalase-peroxidase and small subunit rRNA genes for cyanobacteria with sequenced genomes. Although there are some differences in branching order between the two trees, the marine Synechococcus KatG proteins form a well-supported monophyletic clade, implying that this protein was present in the clade’s ancestor and was subsequently lost in several lineages (indicated by red dots on the rRNA tree), including Prochlorococcus. Green, representatives of the Prochlorococcus clade; orange, marine Synechococcus clade; cyan, other Cyanobacteria. Bootstrap values less than 75% are omitted. Only the tree topologies are shown; branch lengths do not represent genetic distances. Tree construction methods are described in the supplemental material. (Alignments and distance matrices used to produce the figure have been deposited at http://www.datadryad.org [http://dx.doi.org/10.5061/dryad.7j8c5s5j].)
Prochlorococcus relies on the other microorganisms around it to remove hydrogen peroxide from the environment, say the authors, allowing it to fob off its responsibilities on the unlucky card holders around it. This is an instance of how one species can profit from paring down while relying on other members of the community to help out.
So genetic functions provide products that leak in and out of cells and hence become public goods and that is how free-living microorganisms evolve to become dependent on each other, which might be a new way of looking at complicated, inter-dependent communities of microorganisms. The Black Queen Hypothesis could explain how bacteria form biofilms; complex natural communities that often consist of many different kinds of bacteria.
Citation: J. Jeffrey Morris, Richard E. Lenski, and Erik R. Zinser, 'The Black Queen Hypothesis: Evolution of Dependencies through Adaptive Gene Loss', mBio 3:March/April 2012; doi:10.1128/mBio.00036-12






Comments