As portable electronic devices such as laptops and smartphones get more robust, consumers would like for batteries to keep pace. But rechargeable batteries -- Lithium-ion (Li-ion) being among the most popular - haven't done that. They are 25 year old technology powering things produced this year.
Japanese researchers from a prive sector-government team report an advance in Li-ion battery technology that they describe as a major breakthrough. They fabricated a cathode (positive electrode) of lithium cobalt oxide (LiCoO2) in which the compound's individual grains are aligned in a specific orientation. The researchers claim that this yields a significantly higher-performing battery than one with a randomly-oriented LiCoO2 cathode.
Primary, or non-rechargeable, batteries and secondary batteries both produce current through an electrochemical reaction involving a cathode, an anode, and an electrolyte (an ion-conducting material). However, apply an outside current to a secondary battery and the negative-to-positive electron flow that occurs during discharge is reversed. This allows the battery to restore lost charge.
This is a schematic illustration of the crystal structure of LiCoO2.Credit: APL Materials
"In a lithium-ion battery, lithium ions move from the anode to the cathode during discharge and back when charging," said Tohru Suzuki, a co-author on the paper. "The material in the cathode has a layered structure to facilitate intercalation [insertion] of the lithium ions; if the structure is oriented in a specific fashion, the lithium ions have better access to the lattice and, in turn, charge-discharge performance is improved."
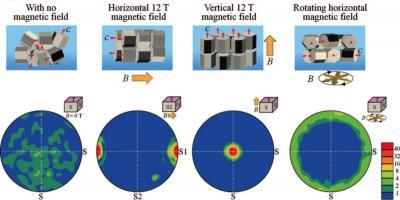
Using a rotating magnetic field, the researchers were able to fabricate the ideal textured microstructure of the individual LiCoO2 grains making up the cathode: a perpendicular alignment of the c-plane (the vertical side) and a random orientation of the c-axis. Unlike cathodes where the microstructures in both the c-plane and c-axis are randomly oriented, the specialized grains allow easy access for lithium ions while relaxing the stress associated with intercalation.
"This yields a highly efficient flow of electrons in both directions," Suzuki said.
Published in APL Materials
http://dx.doi.org/10.1063/1.4824042. Source: American Institute of Physics






Comments