At some point, light bulbs may be one of those things we no longer have to think about but until then the race is on to make a cost-effective bulb that can be used without people grumbling over the cost or the chance of needing a Haz-Mat suit if they break. Researchers at the Universities of Basel and Valencia have described the design of new molecular components and strategies for the preparation of light-emitting electrochemical cells (LECs) with much longer lifetimes than in the past.
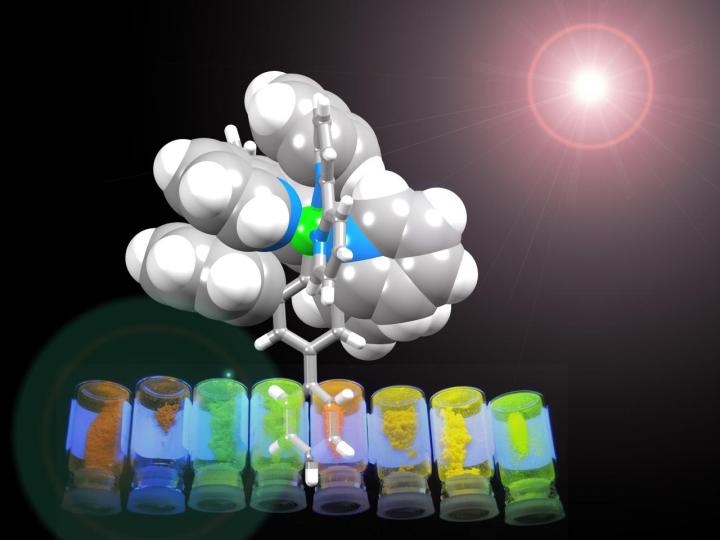
The iridium metal center is wrapped in an organic coat which protects it in the LEC. The precise chemical structure of the coat allows the researchers to tune the color. Credit: © University of Basel
The disadvantage of LEDs is that they are complex, multi-layered devices that require high-vacuum and high temperature techniques for their preparation. They also need to be rigorously protected from exposure to air or water. LECs are much simpler devices, comprising only one layer of active material, which can be solution-processed in ambient conditions, but they are terrifically expensive and have such short lifetimes that they are not a serious alternative for the mass market.
The Basel and Valencia teams created LECs with lifetimes exceeding 2,500 hours using molecular components stabilized by so-called aromatic rings.
The team has built metal complexes decorated with rings that arrange themselves to form a shell around the molecule. "It is a little bit like a flower closing up at night - the flat, petal-like rings fold up about the metal to make a compact and robust structure," says Basel professor Edwin C. Constable.
The supramolecular interactions make the complexes exceptionally stable. Furthermore, molecular tuning of the components allows a tuning of the color of light emitted, bringing the goal of white-light emitting devices one step closer.
Citation: Andreas M. Bünzli, Edwin C. Constable, Catherine E. Housecroft, Alessandro Prescimone, Jennifer A. Zampese, Giulia Longo, Lidón Gil-Escrig, Antonio Pertegás, Enrique Ortí and Henk J. Bolink, 'Exceptionally long-lived light-emitting electrochemical cells: multiple intra-cation π-stacking interactions in [Ir(C^N)2(N^N)][PF6] emitters', Chem. Sci., 2015, 1-10 doi: 10.1039/c4sc03942d





Comments