Proteins, those workhorses of the cell responsible for almost all biological functions, have to be adaptable and that means chain-like molecules must engage in an intricate three-dimensional conformation.
This process - protein folding - is one of the most important in biology. In the event of improper folding, proteins can't perform their duties and may even lump together in aggregates, which can lead to severe diseases like Alzheimer's or Parkinson's.
To prevent that, specialized proteins, so-called chaperones, help other proteins to adopt their proper shape.
The bacterial chaperones GroEL and GroES serve as an example for this principle: together, they build up a cage-like structure in which they encapsulate new, not yet folded proteins, thereby allowing them to fold properly. However, the exact way in which this is accomplished has so far been unclear.
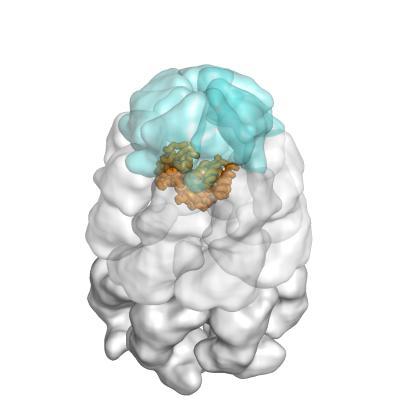
A GroEL/ES nano-cage (light blue and white) with encapsulated substrate protein (orange). Image: Andreas Bracher / Copyright: MPI of Biochemistry
Active acceleration of folding
"Our results demonstrate that the chaperones not only prevent protein clumping, but also dramatically accelerate the folding process." explains Florian Georgescauld, scientist at
Max Planck Institute of Biochemistry
. "Surprisingly, the chaperones achieve this by changing the mechanism of folding: Instead of folding in one large single block, the protein gets its final structure in a series of small, rapid steps – like an elaborate high-speed Origami."
The researchers think that splitting up the reaction might render it energetically more favorable, which in turn would lead to increased speed. Hence, the folding process is finished in a few seconds rather than in several minutes.
The study shows that chaperones can act not only passively, by preventing aggregation, but as an active folding cage that catalyzes the folding process. This results in a high-speed folding mechanism which is of particular biological relevance, so the researchers say, since in this way proteins can be folded faster than they are produced.
Thus, a backlog of proteins which are not yet or improperly folded and the disastrous consequences which might go along with this can be avoided.






Comments