A new computer model believes it may be much faster than previously assumed, as much as 10 times faster. It would also force a rethink for the planet’s unusually high amounts of some isotopes of the elements ruthenium and molybdenum.
The innermost regions of supernovae have helpers floating around: excess protons. Thus, a supernova rich in protons can speed up the triple-alpha reaction, but there is a catch. Accelerating the triple-alpha reaction also puts the brakes on the supernova’s ability to make heavier elements on the periodic table. This is important because scientists have long believed that proton-rich supernovae created Earth’s surprising abundance of certain ruthenium and molybdenum isotopes, which contain closer to 100 protons and neutrons.
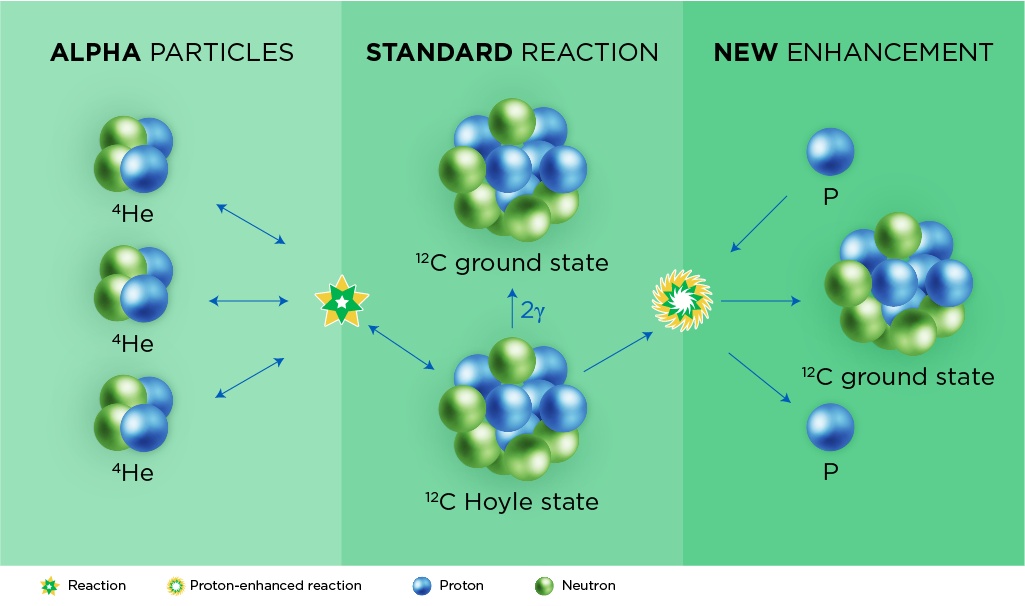
In the triple-alpha process, stars fuse three helium nuclei, alpha particles together to create a single carbon atom with a surplus of energy - a Hoyle state. That Hoyle state can split back into three alpha particles or relax to the ground state of stable carbon by releasing a couple gamma rays (center). Inside supernovae, however, the creation of stable carbon can be enhanced with the help of extra protons (right). Credit: Facility for Rare Isotope Beams
That means ruthenium and molybdenum isotopes aren't being made in proton-rich supernovae, so where? There is speculation but nothing that has created a consensus.






Comments