The ripples generated by chemical reactions, especially when catalyzed - accelerated by substances not themselves consumed - propagate long-range. This challenges the view that molecular motion and chemical reaction reactions affect only the nearby vicinity.
Screening 15 organic chemical reactions, the researchers study chemical reactions that are workhorses with wide application within the organic chemical, pharmaceutical and materials industries. For example, "click" reactions assist the assembly of libraries of biomedical compounds for screening and the “Grubbs” reaction used for plastic manufacture.
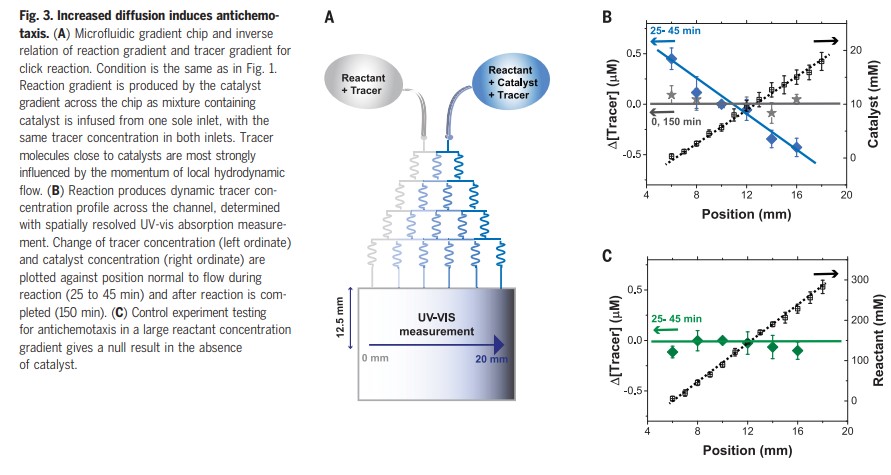
If a majority of all products manufactured require catalysis somewhere in their production sequence, then this could have real-world implications.
In designing their study, the researchers were inspired by noticing that motion can be powered by enzymes and other molecular motors that are prevalent in living systems - but there is no consensus among scientists if these reports could be correctly extended outside biology. Analyzing the problem, the researchers hypothesized that the phenomenon would form an approach to understand molecular machines in the real world.
Testing their hypothesis, the team developed new analytical techniques. Professor Tsvi Tlusty predicted that catalysts in reaction gradients should migrate “uphill” in the direction of lesser diffusivity. Professor Yoon-Kyoung Cho designed a microfluidics chip to test this idea. Dr. Ruoyu Dong, a Research Fellow, performed numerical computer simulations.
The team created guidelines showing that the magnitude of diffusion increase in different systems depends on the energy release rate. These guidelines can be useful practically to estimate the effect in as-yet untested reactions.
To observe that molecules are energized by chemical reaction is “new and unknown,” said Steve Granick, Director of the Institute of Basic Science. “When one substance transforms to another by breaking and forming bonds, this actually makes the molecules move more rapidly. It’s as if the chemical reactions stir themselves naturally.”






Comments