The challenges are doing so at a reasonable cost and having it feel like real meat.
Animal meat consists mostly of skeletal muscle (and fat tissue) which grow in long, thin fibers -- as can be seen in the grain of a steak or when shredding pork or chicken. Reproducing these fibers is one of the biggest challenges in bioengineering meat. Muscle cells are adherent cell types, meaning they need something to hold onto as they grow. To grow muscle tissues that resembled meat, the authors of a recent paper found a 'scaffold' material that was edible and allowed muscle cells to attach and grow in 3D. They did so using a technique known as immersion Rotary Jet-Spinning, which uses centrifugal force to spin long nanofibers of specific shapes and sizes. The team spun food-safe gelatin fibers to form the base for growing cells. The fibers mimic natural muscle tissue's extracellular matrix - the glue that holds the tissue together and contributes to its texture.
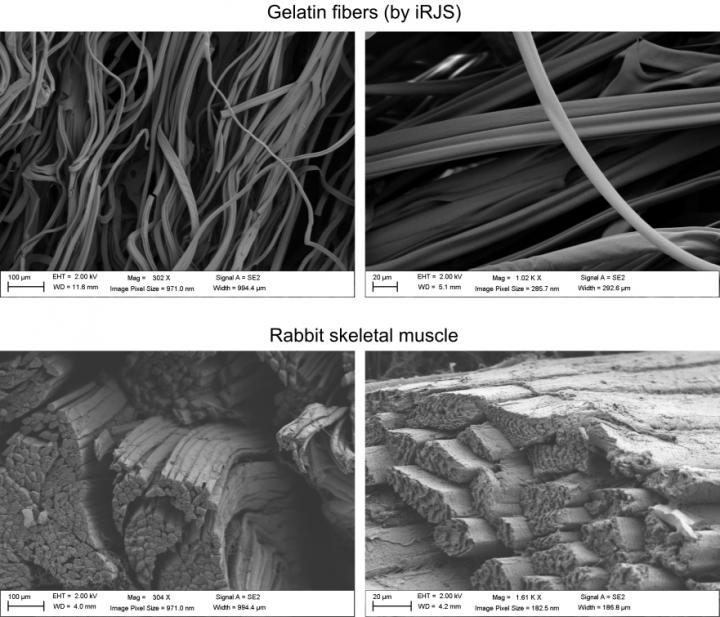
Microscale comparison of gelatin fibers (top) and natural rabbit skeletal muscle (bottom). Scanning electron microscopy images show similar fiber diameter and texture for gelatin scaffolds or skeletal muscle. Credit: Harvard University
The team seeded the fibers with rabbit and cow muscle cells, which anchored to the gelatin and grow in long, thin structures, similar to real meat. The researchers used mechanical testing to compare the texture of their lab-grown meat to real rabbit, bacon, beef tenderloin, prosciutto and other meat products.

You can see the long, thin fibers of skeleton muscle in this close-up of prosciutto ham.
The cultured and natural products had comparable texture but natural meat contained more muscle fibers, meaning they were more mature. Muscle and fat cell maturation in vitro are still a challenge that will take a combination of advanced stem cell sources, serum-free culture media formulations, edible scaffolds, as well as advances in bioreactor culture methods, to overcome.






Comments