We live longer and better than ever and we can thank the continuous advancement of synthetic chemistry, which allows scientists to design and build new molecules.
A new reaction even allows for the edition of organic molecule's skeletons, opening up new avenues of research. In a paper published in the Journal of the American Chemical Society a team present a new reaction able to edit the skeletons of organic molecules by breaking strong C-C double bonds and inserting a carbon atom through a catalytic process.
The researchers present the first catalytic generation of Rh-carbynoids, which emulate the carbene/carbocation behavior of a monovalent cationic carbyne. The catalytic generation of Rh-carbynoids represents a new platform for carbyne transfer that enables skeletal remodelling, and circumvents a long-standing challenge in the catalytic generation of metal-carbynes.
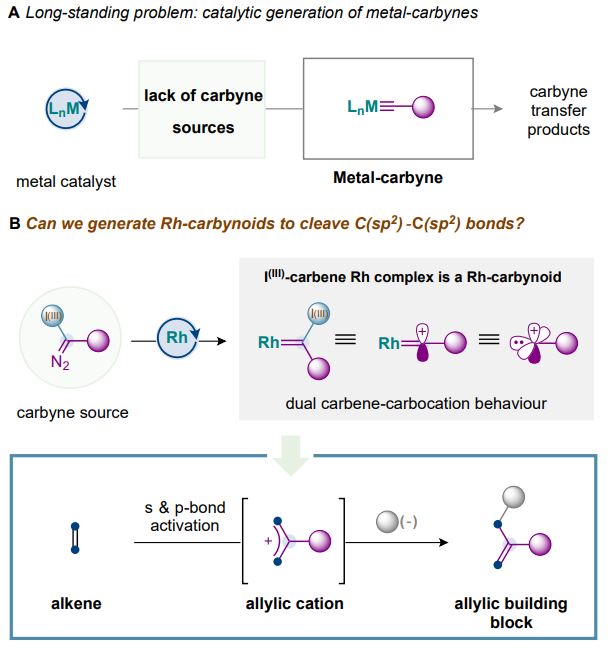
Credit: 10.1021/jacs.9b08632
Aside from inserting a new monovalent carbon atom, the reaction also introduces extra complexity in the molecule: a single C-C bond and a double C-C bond are created together with a chiral center at one of the C atoms with the cleaved double bond.
The skeletal editing will allow building complex architectures, thus expanding the synthetic possibilities of creating new materials or medicines.




Comments