Evolutionary biology sounds exciting - there wouldn't be any movies on the SyFy Channel without Gatoroids and Sharknados and other feats of life science run amok - but in reality you are going to spend a lot of time paying your dues watching sponges in mid-sneeze before you get to create an epidemic or a giant monster.
Sneezing sponges? Isn't that a little far-fetched, even for the network that brought us "Arachnoquake"? No, actually the sponge thing is real, and a new paper points to Porifera sneezing as evidence for a sensory organ in one of the most basic multicellular organisms on Earth, even though it doesn't even have a nervous system to interpret sensory information.
Sponges are pretty simplistic, at least in regards to how we see life. They feed by channeling water through their bodies. They have no digestive or circulatory systems, they live and die by water circulation alone. No noses, because there is no nervous system, so it would be pointless.
Right?
Sponges do sneeze, according to Sally Leys, Canada Research Chair in Evolutionary Developmental Biology at the University of Alberta. It takes about 45 minutes and involves an entire body-wide contraction. It's a sneeze because it reacts to stimuli like we do when we sneeze, in this case something like physical sediment.
Don't believe it? They took this video, one image every 30 seconds, after adding sediment to the water it is filtering.
Credit: Danielle Ludeman
The researchers used a variety of drugs to cause sneezing and then observed the process using fluorescent dye. They focused on the sponge’s osculum, which controls water exiting the organism, including when it sneezes. Their paper says they found that a cellular version of cilia, which function line antennae in other animals, play a role in triggering the sneezes. They concluded that the presence of ciliated cells in the osculum, combined with an apparent sensory function, means the osculum could be a sensory organ.
“For a sponge to have a sensory organ is totally new. This does not appear in a textbook; this doesn’t appear in someone’s concept of what sponges are permitted to have,” said Leys.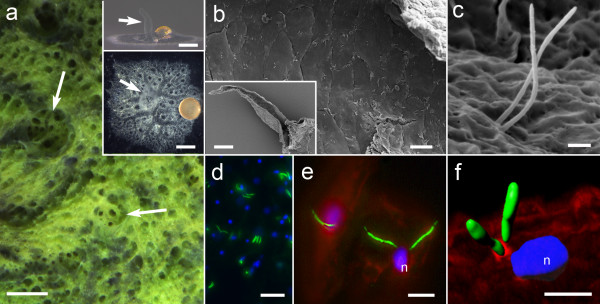
Cilia on the epithelia lining the osculum. a. The sponge Ephydatia muelleri in the lake, and grown in the lab viewed from the side (upper inset) and from above (lower inset). The oscula (white arrows) extend upwards from the body. b, c, Scanning electron micrographs show cilia arise from the middle of each cell along the entire length of the inside of the osculum; b the lining of the osculum with cilia on each cell (inset shows an osculum removed from the sponge and sliced in half longitudinally); c, two cilia arise from each cell. d, e, Cilia in the oscula labeled with antibodies to acetylated α-tubulin (green), nuclei with Hoechst (blue, n), actin with phalloidin (red). f. A 3D surface rendering illustrates how the cilia arise just above the nucleus of the cell. Scale bars a 5 mm; inset 1 mm; b 20 μm; inset 100 μm c, 1 μm d, 20 μm e, f 5 μm. Credit: doi:10.1186/1471-2148-14-3
The fun thing about evolutionary biology is that there are always a lot of new things to learn. This raises new questions about how sensory systems may have evolved. This could be unique and have evolved over 600 million years, or it could be evidence of a common evolutionary history.
“The sneeze can tell us a lot about how the sponge works and how it’s responding to the environment,” said lead author and evolutionary biology graduate student Danielle Ludeman in their statement. “This paper really gets at the question of how sensory systems evolved. The sponge doesn’t have a nervous system, so how can it respond to the environment with a sneeze the way another animal that does have a nervous system can?”
We look forward to the answer.
Citation: Danielle A Ludeman, Nathan Farrar, Ana Riesgo, Jordi Paps and Sally P Leys, 'Evolutionary origins of sensation in metazoans: functional evidence for a new sensory organ in sponges', BMC Evolutionary Biology 2014, 14:3 doi:10.1186/1471-2148-14-3




Comments