
Note: the wire in this photo is oversized to make it easier to see in the photograph.
Wrap the wire around the cylinder to form a loop:
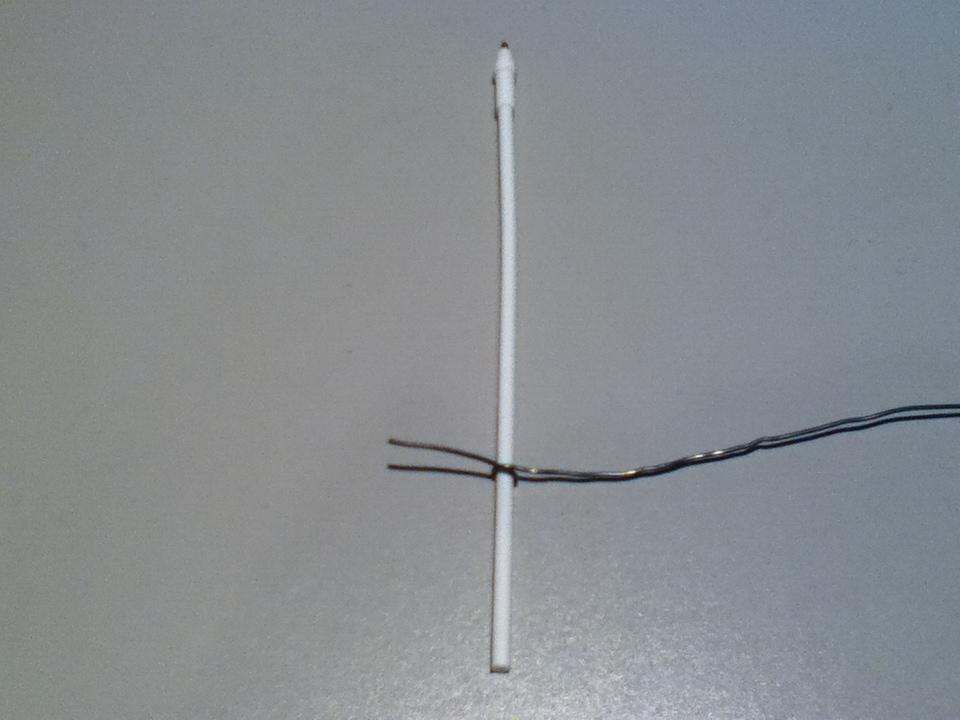
Finally, twist the wire around itself and you now have an inoculating loop:

You can stick your inoculating loop in a cork or use a wooden clothespin for a holder.
To make the borax bead you’ll need some borax (sodium tetraborate) and a flame from an alcohol lamp or stove. Put a few grams of borax on a nice clean surface—you can use a blank sheet of computer printer paper, watch glass, or evaporating dish. Heat the inoculating loop in your flame until the loop is red hot. Then dip the loop in the borax. This will coat the loop in borax. Put the loop back into the flame. The borax will swell and then shrink to a white, glassy bead. It may take a few tries, but repeat the process until you have a bead that is at least 3 mm in diameter.
You can use two parts of the flame for the bead tests—the outer cone will be the oxidizing portion of the flame and the inner portion of the flame will be the reducing cone. Coat the bead with sodium chloride, or ordinary table salt (NaCl) and then place the bead in the reducing flame and see what color it makes. Repeat the process in the oxidizing flame. For a table of colors that you’ll see with different metals, see my source. To have some fun making colored borax beads, see this source .
You can also use your inoculating loops without the borax bead to perform flame tests. First, clean the loop by dipping it in hydrochloric acid (HCl, sold as muriatic acid) and then rinse it with deionized or distilled water. Dip the loop in the table salt and place it in the reducing cone of the flame. Repeat the process in the oxidizing cone.
You can use cotton swabs as well for flame tests. Dip a clean swab in distilled water and then dip the swab in your table salt and place it in the reducing/oxidizing cone.
There are a number of resources for making different colored flames using chemicals as powders or solutions, such as this site, but you can use your Google-fu to find others.
--------------------
For more information about the Science Play and Research Kit (SPARK):
For more articles regarding SPARK, type "science play and research kit" in the search box above and click the "Go" button.



Comments