"The model we present shows how simple evolutionary mechanisms can cause the extinction of populations of fast mutating pathogens, such as certain viruses", co-author of the study and Centre of Astrobiology researcher Susanna C. Manrubia explained to Servicio de Información y Noticias Científicas (SINC).
The results of the research, which have been published this year in Europhysics Letters, suggest that strategies can be devised to fight viral infections by gaining a better understanding of their population dynamics. A moderate increase in the mutation rate of such viruses could become a therapy alternative to the massive use of drugs.
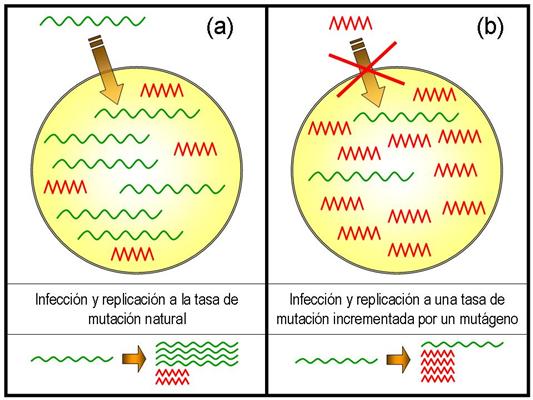
Representación esquemática de la composición de una población de virus en el interior celular, con formas viables (en verde) y no viables (en rojo), con dos tasas de mutación distintas. En (a), con una tasa de mutación natural, las formas viables son mayoría. Las formas no viables, que no pueden infectar nuevas células, se originan cuando las viables se replican con errores, y también son capaces de auto-replicarse. En (b) la tasa de mutación aumenta (añadiendo un mutágeno, por ejemplo) y las formas no viables pueden volverse mayoritarias, favoreciendo la desaparición de las formas viables, momento en que la población en general pierde su capacidad infectiva. Imagen: Manrubia et al
The scientists experimented with the lymphocytic choriomeningitis virus (LCMV), which produces persistent infections in house mice and is sometimes transmitted to humans. This virus does not normally cause serious problems, but occasionally results in death among people with a weak immune system or abortion if infection occurs during pregnancy.
"The high mutation rates of these viruses allow them to maintain a reservoir of variants so as to adapt to possible environmental changes and to challenges such as immune system attacks on behalf of the host or target cell heterogeneity," Manrubia says.
However, this high rate of mutation also produces a high number of unviable mutants, capable of surviving at the expense of viable forms. In order to create this situation and raise the natural rate at which viruses mutate, scientists add mutagen. In the case of LCMV, fluorouracil is used.
By adding mutagen, the ability of the virus to infect cells disappears, although its replicative ability is not affected. The researchers believe that this occurs because the number of unviable mutants, which can replicate but not infect, act "like a cancer" that destroys the system from the inside.
"The mathematical model formally characterizes the extinction of infectivity in these viruses following experimental results and demonstrates three things: this occurs with small amounts of mutagen, which is much more likely if there is only a small number of viral genomes inside a cell and, most importantly, it is a new mechanism for viral extinction that could potentially have clinical uses in the medium term" Manrubia says.
Manrubia developed the model alongside Jaime Iranzo, who joined the Centre of Astrobiology recently. Iranzo received the Archimedes Prize for the best research paper in the field of physics for this study. The prize is awarded by the Spanish Ministry of Science and Innovation to university students or recent university graduates.
Article: Jaime Iranzo, Susanna C. Manrubia, “Stochastic extinction of viral infectivity through the action of defectors”, Europhysics Letters 85 (1): 18001(p1-p6), 2009.




Comments