They'll be too late to help with the actual COVID-19 but since coronavirus constantly mutates, like the flu, and 2019 was the third coronavirus pandemic in the last 17 years, it could be valuable for the next iteration.
One strategy employs modified bacteriophage particles that can be inhaled to deliver protection via the lungs to the immune system. The other delivers injectable adeno-associated virus-phage particles that directly encode protection against the virus in immune cells. They're only in rodents so far but they produced antibodies.
The phage particles are engineered with an epitope from the SARS-CoV-2 spike protein, along with a small ligand peptide that helps the phage cross from the lungs into the recipient’s bloodstream. Once there, they essentially teach the immune system to guard against COVID-19. An advantage to a phage-enabled vaccine is that one spike protein can carry a multitude of epitopes, and they can be easily customized to protect against COVID-19 variants evolving now and thus can be modified for future coronavirus pandemics as well.
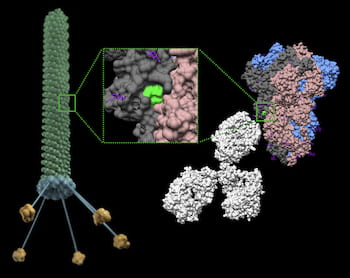
In this graphic, a phage particle displays a region of the SARS-CoV-2 spike protein that retains a near-native structural conformation. Administration of this phage in mice induces a systemic immune response against the spike protein. This versatile technology serves as a first step towards developing cost-effective and easy-to-manufacture vaccines for COVID-19 and other infectious agents. Illustration by Christopher Markosian/Daniela Staquicini/Rutgers Cancer Institute of New Jersey/Rutgers New Jersey Medical School
In experiments with rodents, Epitope 4 delivered the best immune response by nearly a factor of 10 over the other candidates. With that, they need to expand the search for more and better epitopes.
“We will try to computationally screen the entire surface of the spike protein to find new epitopes,” said Rice physicist José Onuchic, a co-principal investigator on the project. “If you just do this by trial and error, by brute force, it’s going to be too expensive or too long a process. By computational screening, we can come up with a short list of new candidates.”
This may be new for academia but the private sector has routinely modeled new drugs and then went into animal testing for decades. While rodents are not little people, no study in rats or mice can ever show benefit or harm in humans, they can eliminate benefit or harm in humans. That is why all approved drugs survived clinical trials after animal tests before getting to market.
A single vaccine that protects against multiple COVID-19 variants and can be transported without the “cold chain” required for current vaccines will be key to finally curtail the worldwide epidemic, said Renata Pasqualini, who co-led the study.






Comments