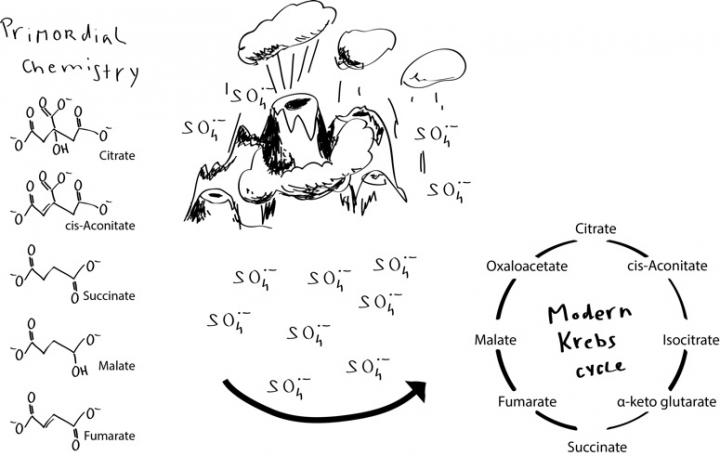
One central metabolic pathway learned by every A-level biology student is the Krebs cycle - which Nobel laureate Krebs actually called the citric acid cycle. It utilizes Coenzyme A as the first step in our body's energy factory products. But how did this essential set of chemical reactions, each step catalyzed by an enzyme, first arise? Each step in the cycle is not enough by itself. Life needs a sequence of these reactions, and it would have needed it before biological enzymes were around: Amino acids, the molecular components of enzymes, are made from products of the Krebs cycle.
A new paper demonstrated a network of chemical reactions in the lab which mimic the important Krebs cycle present in living organisms today, and say it could explain an important step in how life developed on Earth.
A set of biochemical processes crucial to cellular life on Earth could have originated in chemical reactions taking place on the early Earth four billion years ago. Credit: Aleksej Zelezniak/Francis Crick Institute
Life developed four billion years ago on a harsh, volcanic Earth that lacked any oxygen, but that did possess large oceans rich in metal ions. There has been much interest in how the first life forms developed in these conditions and how the biochemical processes necessary to sustain life could form from nothing. The research group from the Francis Crick Institute and the University of Cambridge say their demonstration offers an answer. They have shown an enzyme-free metabolic pathway that mirrors the Krebs cycle. It is sparked by particles called sulphate radicals under conditions similar to those on Earth four billion years ago.
The scientists used simple carbon compounds which are involved at various points in the Krebs cycle (such compounds have recently been found in a meteorite by NASA scientists) and mixed them with iron and sulphur-containing chemicals that would be found in sediments in the early oceans.
They carried out a systematic screening strategy of around 4,850 different experiments using mass spectrometry techniques, and looked out for reactions similar to those seen in the Krebs cycle.
In the vast majority of cases, the mixtures were unreactive. However, in the presence of the compound peroxydisulfate, the researchers detected 24 chemical reactions. These resembled the pattern of reactions seen in the Krebs cycle in living organisms.
An alternative hypothesis for the origin of life suggests that RNA - a molecule similar to DNA that can maintain genetic information but is more transient and more reactive - can explain the first steps towards life. This is known as the RNA-world hypothesis but the presence of RNA molecules cannot easily explain the origin of metabolism, as RNA is made from products of metabolism. The new results support the hypothesis that environmental chemistry enabled metabolism to begin.






Comments