#2 in a series.
Sir William Ramsay wrote an excellent history of the study of our atmosphere. Below is the part of his book concerning John Mayow. The previous part was about Robert Boyle. The text courtesy archive.org. is error-checked for typos.

The earliest accounts of the reduction in volume of a quantity of air during combustion are due to Philo of Byzantium (ca. 280 - 220 BC) and Leonardo da Vinci (1452 – 1519). John Mayow went much further with his experiments.
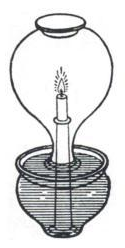
Philo's experiment
image courtesy Wikipedia.
Before modern scientific knowledge of the atmosphere there came a hard-won knowledge of its chemical composition. John Mayow was an early researcher into that component of the atmosphere which is essential to animal life - he called it 'nitro-aerial particles', 'igno-aerial particles' or 'fiery air', we call it oxygen. Note in particular his use of the term 'particles', presaging John Dalton's atomic theory.
The reason why Mayow is so little known in the history of chemistry and atmospheric science is explained in these extracts from the main text :
His treatise on respiration "was inserted in an abridged form in the Philosophical Transactions of the Royal Society some time after its publication, but received only scant recognition, for the fame of Newton and Boyle overshadowed the labours of less well-known investigators. And Mayow did not live to press his discoveries on the attention of his contemporaries, for he died in October 1679, five years after the publication of his tracts, in his thirty-seventh year."
"His scientific work proves that if he had been granted the usual span of life, his extraordinary genius would have furthered the knowledge of the true explanation of the nature of air, and its function in supporting combustion and respiration, and that his views would have been accepted more than a century before Lavoisier..."
The Gases of the Atmosphere - the History of Their Discovery
By Sir William Ramsay, K.C.B., RR.S.
Fourth edition 1915
Courtesy archive.org.
CHAPTER I
THE EXPERIMENTS AND SPECULATIONS OF BOYLE, MAYOW, AND HALES
John Mayow
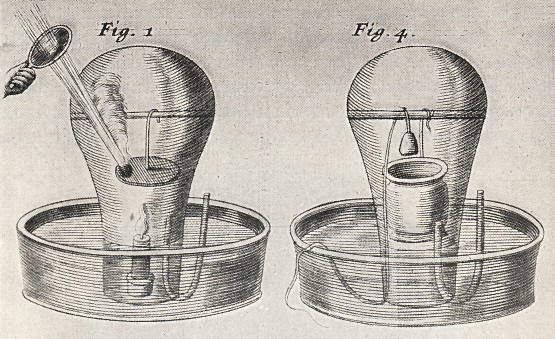
Mayow's burning experiment
image courtesy Wikipedia
John Mayow was born in the parish of St. Dunstan in the West, London, in 1643. His family was originally Cornish, having come from Bree, in Cornwall. He entered Wadham College, Oxford, on April 3rd, 1658, at the early age of fourteen, and was shortly afterwards made a probationer-fellow of All Souls College. On May 30th, 1665, after nearly seven years of study, he took the degree of B.A. ; in 1670, he graduated as Doctor of Laws (LL.D.) ; but not being attracted by the legal profession, he turned his attention to medicine, and became a medical practitioner at Bath, where he lived during the fashionable season. When not more than twenty - five years of age, he wrote two essays on respiration, ascribing the inflation of the lungs to the action of the intercostal muscles. These " Tractatus duo " were published in 1668. Some years later he produced the treatise on which his fame rests; it is entitled " Tractatus quinque medico-physici, quorum primus agit de sal-nitro et spiritu nitro-aereo; secundus, de respiratione ; tertius, de respiratione foetus in utero et ovo ; quartus, de motu musculari, et spiritibus animalibus ; ultimus, de rhachitide ; studio Joh. Mayow, LL.D.&Medici, nec non Coll. Omn. Anim. in Univ. Oxon. Socii. Oxonii e Theatro Sheldoniano, An. Dom. MDCLXXIV." The work was dedicated to Mr. Henry Coventry. It was inserted in an abridged form in the Philosophical Transactions of the Royal Society some time after its publication, but received only scant recognition, for the fame of Newton and Boyle overshadowed the labours of less well-known investigators. And Mayow did not live to press his discoveries on the attention of his contemporaries, for he died in October 1679, five years after the publication of his tracts, in his thirty-seventh year. Little is known of Mayow's domestic life, save that he married shortly before his death. His scientific work proves that if he had been granted the usual span of life, his extraordinary genius would have furthered the knowledge of the true explanation of the nature of air, and its function in supporting combustion and respiration, and that his views would have been accepted more than a century before Lavoisier with fuller knowledge, and with the scientific position which at once gained a hearing forced precisely similar doctrines upon the attention of the scientific world. Mayow was a contemporary of Boyle, and frequently made use of Boyle's experiments in support of the theories which he advanced. Curiously enough, while Boyle seems to have read Mayow's work, he does not appear to have been favourably impressed by his conclusions. Boyle, at the age of fifty-two, had doubtless formed his own opinions, and was unwilling that they should be disturbed by the speculations, well founded though they were, of so young a man. And shortly after Mayow's death, the views of Becher, one of his contemporaries, expounded and made definite by Stahl, regarding the nature of combustion, were universally received. After Lavoisier's theories had overthrown these false views, attention was again directed to Mayow's tracts, first by Blumenbach, in his Institutiones Physiological; later by T. Beddoes, in 1790, who wrote a digest of Mayow's work under the title "Chemical Experiments and Opinions extracted from a Work published in the Last Century " ; and later by Johann Andreas Scherer, in a work published at Vienna in 1793, and also by Dr. Yeats in 1798. Scherer gives a careful analysis of Mayow's work, somewhat altering the order of his paragraphs, with a paraphrase in German of the Latin text, which he quotes in full. Yeats' treatise is more especially concerned with the medical aspect of Mayow's work, although it also deals with the purely chemical portion at considerable length. 1 In the following account of Mayow's researches free use has been made of both of these works, as well as of his own " Tracts." Mayow's contributions to the chemistry of the atmosphere may be classified thus :
1. The atmosphere consists of particles of two kinds of gas at least : one of these, termed " nitro- aerial particles," is necessary for the support of life and for the combustion of inflammable bodies ; while the other, left after this constituent has been removed, is incapable of supporting either life or combustion. The portion which is necessary for life enters, during respiration, into the blood. It is the chief cause of motion in animals and in plants.
2. These " nitro - aerial particles " are also present in saltpetre or nitre, as can be shown by mixing inflammable substances, such as sulphur and charcoal, with nitre to form gunpowder, filling a tube with the powder, and, after setting it on fire, immediately plunging the open end of the tube under water. The sulphur and charcoal will be as completely consumed as if burned in the open air. Such combustion might, however, be ascribed to a " sulphureous " constituent in saltpetre ; by "sulphureous" is to be understood combustible, for those substances capable of burning were imagined to contain a " sulphur " which gave them that property. That nitre does not contain such " sulphur" can be shown by exposing it alone to heat, when no change takes place, except fusion. Besides, nitre is compounded of " spirit of nitre " or nitric acid and pure alkali, neither of which contains a combustible sulphur; hence the particles of fire-air must be present in nitre in no small amount. But it is probable that it is the spirit of nitre which contains such fire-air particles, because, as will be shown later, they are not present in the alkali.
One difficulty occurs to Mayow. How is it that so large a quantity of gas as is necessary to support combustion can be contained in a relatively small bulk of saltpetre ? He tries whether a solution of saltpetre evolves air -bubbles when placed in a vacuum, and finds that it effervesces less than pure water does. He also prepares saltpetre by mixing nitric acid and alkali in a vacuum ; a brisk effervescence occurs, and the dried- up salt is ordinary saltpetre. Hence saltpetre cannot contain elastic air. Mayow consequently draws a distinction between "air" and " air-particles."
The residue left after the " fire-air," or spiritus igneo-aerius, has been removed from ordinary air by breathing or by combustion is proved to be lighter than the fire-air itself; because a mouse dies sooner if kept at the top of air in a confined bell-jar than at the bottom ; and a candle goes out sooner. Here the conclusion is right, although the reason given is wrong ; for it is the temperature of the respired air which makes it rise, and not the fact that it is specifically lighter than the oxygen.
Metallic antimony gains in weight when it is set on fire by a lens, and burns ; if this gain in weight, Mayow remarks, is not due to the absorption of nitro-aerial particles and to the fire, it is difficult to say to what it is due.
The reason why substances burn so violently in nitre compared with air, is because of the proximity of the fire-air particles; and these are evidently due to the nitric acid, because the residue, the alkali, if mixed with sulphur and inflamed, does not produce ignition.
2. All acids contain fire-air particles, for acids have great similarity to each other. This is shown as follows : Antimony made into a calx by the sun's rays with a burning-glass gives the same calx as when it is evaporated repeatedly with nitric acid and converted into " Bezoar-mineral," i.e. oxide of antimony. And iron-rust obtained from sulphide of iron appears to be formed by the union of the fire-air particles with the metallic " sulphur " of the iron.
It has up till now been believed that sulphuric acid is an ingredient of common sulphur. But this is unlikely, for sulphur has a sweetish, and not an acid taste. Moreover, quite a different substance from a vitriol (or sulphate) is obtained by melting together alkali and sulphur ; and no effervescence takes place during its preparation. Sulphur, too, is precipitated out of the "liver of sulphur" (potassium persulphide) by the addition of sulphuric acid. Now, were sulphuric acid contained in sulphur, it would hinder the union of the sulphur with the alkali.
It is to be noticed that the volatile sulphuric acid, from the combustion of sulphur, is produced in the following way : " The flame of the burning sulphur consists, like every other flame, in the violent motion of the sulphur particles with that of the nitro-aerial particles ; hence the sulphur particles, at first solid, become sharp and acid, and probably form the ordinary ' spirit of sulphur ' (sulphuric acid). If this be not so, I know not in what manner this acid can be produced ; for, as has been shown, it is very improbable that it previously existed in the mass of the sulphur before its deflagration. Such a change also, in all probability, takes place in pyrites, when it is converted to green vitriol ; because pyrites yields sulphur on distillation; and the green vitriol on distillation gives sulphuric acid, leaving red colcothar (iron oxide) behind."
Similarly, nitre appears to be a triple salt, formed by the union of the fiery part of air with a salt-like substance existing in the earthy material, together forming nitric acid ; and this, added to earthy salts (alkali), yields ordinary nitre. " I have tried to show that all acids consist of certain saline particles rendered fluid by the nitro-aerial particles.'
4. Boyle has shown that a flame is extinguished more rapidly in a vacuous space than in a confined space containing air ; this is obviously due to absence of nourishment in the air, rather than to its choking by its own vapours ; for in the vacuous vessel there is evidently more space for such noxious vapours than in the air-filled vessel, and yet the flame is more rapidly extinguished. Moreover, no combustible matter can be kindled in a vacuum by means of a burning-glass. But it must not be concluded that this fire-air constitutes the whole of ordinary air ; because a candle goes out in air confined in a glass while a large quantity of air is still contained in it.
While gunpowder burns owing to the fire-air particles which it contains, and requires no sustenance from external air, the combustion of vegetables is supported partly by the igno-aerial particles which they themselves contain, partly by those of the external air.
Air which has supported combustion loses to some extent its elasticity (i.e. diminishes in volume), as shown by the burning of a candle in air confined over water. This is to be ascribed partly to actual loss of elasticity, partly to the absorption of the fire-air. The loss of volume amounts to about three per cent of the whole quantity of air taken.
All this is exceedingly clear, and in accordance with our modern views, but Mayow's mind is somewhat confused with reference to flame and heat, since he imagined that the diminution of the volume of air in which combustible substances have been burned is due to the escape of heat ; and inasmuch as a rise of temperature was known to increase the volume of air, so a loss of heat should, in his opinion, produce the opposite effect. The fire-air particles are apparently regarded as a sort of compound of heat with matter (as indeed in a certain sense they are) ; and by combustion or by respiration both are removed. The loss of volume is to be explained by the removal of both from the air, and the gain in weight by the union of the matter with the combustible body, such as antimony.
Such is a brief account of Mayow's views on the nature of atmospheric air. But the tale would be incomplete without mention of the fact that he prepared a gas by the action of nitric acid on iron, viz. nitric oxide, which, when introduced into ordinary air confined over water, decreased its volume; and he found that further admission of nitric oxide produced no further diminution in the volume of the air. A very little more, and he would have recognised in this a means of analysing air, and depriving it wholly of its oxygen. He goes so far as to speculate that a compound is formed between the nitric oxide and the oxygen, but the solubility of gases in water appears not to have struck him as important. He notices, however, that the combination of the two gases is attended by rise of temperature, and is in so far analogous to combustion.
It would lead us too far to consider in detail Mayow's theories of fermentation and of respiration. Suffice it to say that he ascribes the production of animal heat to the consumption of his fire-air particles by the animal, and remarks that the pulse is heightened by respiration. This view was in opposition to that held by his contemporaries, viz. that the purpose of respiration was to cool the blood.
It is impossible to avoid being impressed with the clearness and justice of Mayow's inferential reasoning. All that was wanting was the discovery of oxygen and carbon dioxide, and the identification of the first with his fire-air, and of the second with one of the products of combustion. But these discoveries were not made until a century after his death. Had he lived, there can be little doubt that, unless discouraged by the want of appreciation with which his ideas were received, he would have continued to labour in the fruitful fields from which he had already reaped so rich a harvest.
Before leaving the seventeenth century, it is perhaps fitting to mention the name of Jean Key, a French physician, who wrote in 1630 concerning the gain in weight of tin and lead when calcined. While Key exhibited some leaning to wards the modern methods of experimentation, he still lay fettered in the bonds of mediaeval scholasticism. In discussing the weight of air and fire, he finds occasion to consider the question whether a vacuum can exist. His words are so quaint that they are worth quoting : " It is quite certain that in the bounds of Nature a vacuum, which is nothing, can find no place. There is no power in nature from which nothing could have made the universe, and none which could reduce the universe to nothing : that requires the same virtue. Now the matter would be otherwise if there could be a vacuum. For if it could be here, it could also be there ; and being here and there, why not elsewhere ? and why not everywhere ? Thus the universe could reach annihilation by its own forces ; but to Him alone who could make it is the glory of being able to compass its destruction." And since air cannot be drawn down by a vacuum, it must descend by virtue of its own weight when it fills a hole. And hence, as air has weight, tin and lead gain in weight when they combine with air. It will be admitted that this is very inferior to the speculations and deductions of Boyle and Mayow.
-----------------------------------------------------------------------------------------
footnotes
1 An English translation of Mayow's chemical researches has been published by Prof. Crum Brown in the Alembic Club Reprints (James Thin, Edinburgh, 1907).
Philo of Byzantium described what was surely the world's first washing machine: a rather Goldbergian contraption.
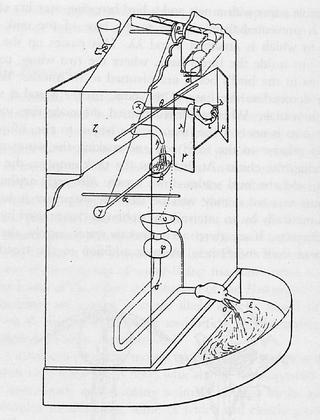
image courtesy Wikipedia
.





Comments