that response to aggressive chemotherapy and radiation differed by subtype.The research team for TCGA is a collaborative effort funded by the National Cancer Institute (NCI) and the National Human Genome Research Institute (NHGRI), both parts of the National Institutes of Health. The Cancer Genome Atlas (TCGA) aims to catalogue and discover major cancer-causing genome alterations in large cohorts of human tumours through integrated multi-dimensional analyses.(3)The study, published Jan. 19, 2010 in Cancer Cell, provides a solid framework for investigation of targeted therapies that may improve the near uniformly fatal prognosis of this
 cancer.Although the findings do not affect current clinical practice, the researchers said the results may lead to more personalized approaches to treating groups of GBM patients based on their genomic alterations. (1,2)
cancer.Although the findings do not affect current clinical practice, the researchers said the results may lead to more personalized approaches to treating groups of GBM patients based on their genomic alterations. (1,2)Subtypes of GBM
The four subtypes of GBM identified were Proneural, Neural, Classical, and Mesenchymal.NF1 mutation and loss define Mesenchymal GBM while focal EGFR (epidermal growth factor receptor) events define Classical GBM and PGFRA (platelet-derived growth factor)\IDH1 (Isocitrate dehydrogenase 1 )events define Proneural GBM.Gene signatures of normal brain cell types show a strong relationship between subtypes and different neural lineages. Additionally, response to aggressive therapy differs by subtype, with the greatest benefit in the Classical subtype and no benefit in the Proneural subtype.The reproducibility of this classification was demonstrated in an independent validation set, suggesting that it is highly unlikely that these GBM tumor subtypes are a spurious finding due to technical artifact, chance, or bias in TCGA sample qualification criteria.The researchers wrote that larger sample set might describe additional subtypes for which they lack the power to detect and provided the community with the means to identify the tumor subtypes prospectively (http://tcga-data.nci.nih.gov/docs/publications/gbm_exp/). (1)
Subtypes and Treatment Effects
An association was observed between the Proneural subtype and age with it appearing to affect younger people more and also a trend toward longer survival. The data also suggested that
Proneural samples do not have a survival advantage from aggressive treatment protocols. Importantly, a clear treatment effect was observed in the Classical and Mesenchymal subtypes. Profiling-based classification may therefore have highest clinical relevance in suggesting different therapeutic strategies.(1)
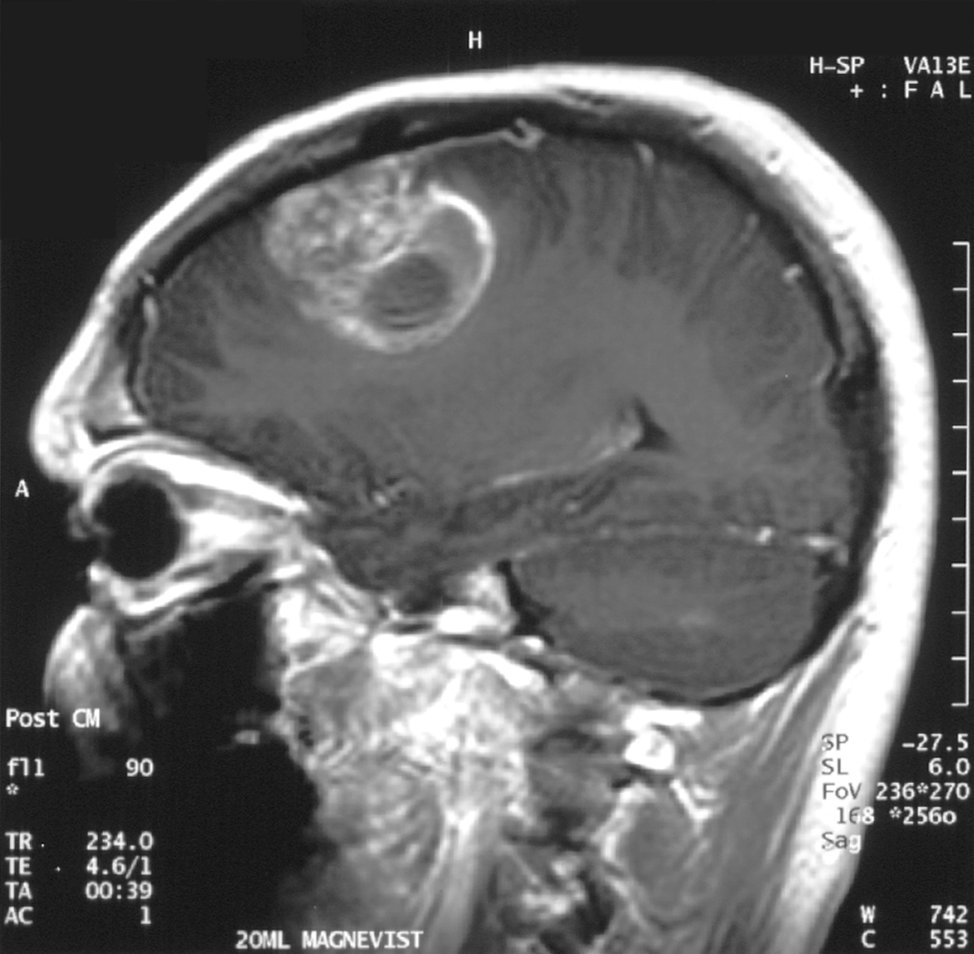
Glioblastoma Multiforme
GBM is a very fast-growing type of tumor. In recent years, three of every 100,000 Americans have been diagnosed with GBM, representing the highest incidence rate among malignant brain tumors. While GBM comprises approximately 25% of all brain tumors in adults, in absolute numbers it is still an uncommon cancer.Patients with newly diagnosed glioblastoma have a median survival of approximately 1 year with generally poor responses to all therapeutic
modalities.(1,2,3)
 Primary glioblastoma, which comprises more than 90% of biopsied or resected cases, arises de novo and present initially as grade 4 tumors while secondary GBMs present as lower grade gliomas and progress to GBMs over time.Two decades of molecular studies have identified important genetic events in human glioblastomas which include (1) dysregulation of growth factor signalling by amplification and mutation of receptor tyrosine kinase (RTK) genes; (2) activation of the phosphatidylinositol-3-OH kinase (PI(3)K) pathway; and (3) inactivation of the p53 and retinoblastoma tumour suppressor pathways (p53 and retinoblastoma pathway are involved in regulation of the cell cycle).(3,4)
Primary glioblastoma, which comprises more than 90% of biopsied or resected cases, arises de novo and present initially as grade 4 tumors while secondary GBMs present as lower grade gliomas and progress to GBMs over time.Two decades of molecular studies have identified important genetic events in human glioblastomas which include (1) dysregulation of growth factor signalling by amplification and mutation of receptor tyrosine kinase (RTK) genes; (2) activation of the phosphatidylinositol-3-OH kinase (PI(3)K) pathway; and (3) inactivation of the p53 and retinoblastoma tumour suppressor pathways (p53 and retinoblastoma pathway are involved in regulation of the cell cycle).(3,4)Implications
"These new findings offer critical insights into stratifying patients based on the unique molecular characteristics of their disease," said John E. Niederhuber, M.D., NCI director. "As we learn
more and more about the genetic underpinnings of cancer, we hope to achieve a similar level of molecular understanding for all cancers and eventually to generate recipes of highly targeted therapies uniquely suited to the individual patient."(3)
Because the response to aggressive chemotherapy and radiation differed by subtype, some classes of drugs would be expected to work for some tumor subtypes and not others.(3)"The ability to differentiate GBM tumors based on their altered genetic code lays the groundwork for more effective treatment strategies to combat this deadly cancer," said Eric D. Green, M.D., Ph.D., NHGRI director. "These findings demonstrate the power of using a cancer's genome to unravel the molecular changes that occur in the various cancer types targeted by TCGA. I'm optimistic that this type of knowledge will someday lead to improved personalized therapies and care for cancer patients."(3)
include a molecular test for subtype and linked molecular genetics for key genetic events, including NF1 and PTEN (phosphatae and tensin homolog) loss, IDH1 and PI3K mutation, PDGFRA and EGFR amplification (i.e., genetic events that are best assayed on the DNA level), and MGMT (O-6-methylguanine-DNA methyltransferase encoded by MGMT gene)methylation status. Methylation is the the addition of a methyl group to DNA and essential for normal development with it being associated with a number of key processes including genomic imprinting, X-chromosome inactivation, suppression of repetitive elements and carcinogenesis. In addition, early evidence suggests that subclasses differ measurably by signal transduction pathways such that
protein biomarkers might be easily measured (4). Future studies should further elucidate the intricate relationship between tumor subtypes, treatment sensitivity, and MGMT methylation status.(1)
Further Information
TCGA data are being made rapidly available to the research community through a database, http://cancergenome.nih.gov/dataportal.The database provides direct access to most analytic datasets, with other data, such as patient treatment records, available to qualified researchers through an NIH review and approval process.The TCGA Research Network consists of more than 150 researchers at dozens of institutions across the nation. A full list of participants is available at http://cancergenome.nih.gov/wwd/program.
More details about The Cancer Genome Atlas, including Quick Facts, Q&A, graphics, glossary, a brief guide to genomics and a media library of available images can be found at http://cancergenome.nih.gov.NCI leads the National Cancer Program and the NIH effort to
dramatically reduce the burden of cancer and improve the lives of cancer patients and their families, through research into prevention and cancer biology, the development of new interventions, and the training and mentoring of new researchers. For more information about
cancer, please visit the NCI Web site at http://www.cancer.gov or call NCI's Cancer Information Service at 1-800-4-CANCER (1-800-422-6237).
NHGRI supports the development of resources and technology that will accelerate genome research and its application to human health. Additional information about NHGRI can be found at its Web site, http://www.genome.gov.The National Institutes of Health (NIH) — The Nation's Medical Research Agency — includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. It is the primary federal agency for conducting and supporting basic, clinical and translational medical research, and it investigates the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov.
- Verhaak RGW, Hoadley KA, et al. Integrated Genomic Analysis
Identifies Clinically Relevant Subtypes of Glioblastoma Characterized
by Abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell, Jan 19, 2010 DOI: 10.1016/j.ccr.2010.12.020. - http://www.eurekalert.org/pub_releases/2010-01/nhgr-tcg011510.php
3.Cancer Genome Atlas Research Network (2008)'Comprehensive genomic characterization defines human glioblastoma genes and core pathways' Nature 455, 1061-1068 (23 October 2008) doi:10.1038/nature07385
4.Brennan, C, Momota,h et al(2009) PLoS One. 2009; 4(11): e7752. (13 November 2008) .doi: 10.1371/journal.pone.0007752.
Further Reading
- Diagram of PI3K/Akt Signaling pathway :http://www.cellsignal.com/pathways/akt-signaling.jsp





Comments