We hear a lot about carbon storage and the impact on the atmosphere if CO2 is release during warming, but how does that work?
Carbon is not evenly distributed in soil, instead the kinds of carbon hot spots that matter are found on about 20 percent of mineral surfaces, according to a new paper. Studies have established that carbon binds to tiny mineral particles and in a new paper researchers show that the surface of the minerals plays just as important a role as their size.
"The carbon binds to minerals that are just a few thousandths of a millimeter in size – and it accumulates there almost exclusively on rough and angular surfaces," explains Prof. Ingrid Kögel-Knabner,
Technische Universitaet Muenchen
Chair of Soil Science.
They believe that the rough mineral surfaces provide an attractive habitat for microbes. These convert the carbon and play a part in binding it to minerals.
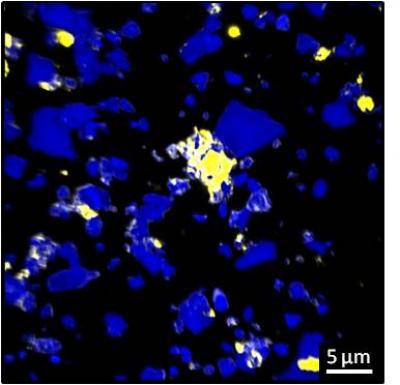
Carbon tends to bind to specific rough mineral surfaces, known as hot spots (yellow areas). Credit: C. Vogel/TUM
The role of microorganisms in sequestering carbon
"We discovered veritable hot spots with a high proportion of carbon in the soil," relates Cordula Vogel, the lead author of the study. "Furthermore, new carbon binds to areas which already have a high carbon content."
"Thanks to our study, we can now pin-point the soil that is especially good for sequestering CO2," says Kögel-Knabner. "The next step is to include these findings in carbon cycle models."
The sample material used by the team was loess, a fertile agricultural soil found in all parts of the world – which makes it a very important carbon store. The researchers were able to take ultra-precise measurements using the NanoSIMS mass spectrometer. This procedure allowed them to view and compare even the most minute soil structures.
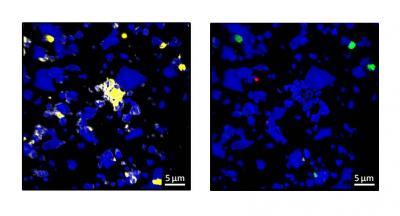
Left: Mineral surfaces with all accumulations of carbon (yellow). Right: Mineral surfaces with new organic substance (green and magenta). Credit: C. Vogel/TUM






Comments