Researchers trying to get new information about the metabolism of plants can switch off individual genes and study the resulting changes but researchers in a new study adopted a different approach.
Erich Kombrink from the Max Planck Institute for Plant Breeding Research in Cologne and Markus Kaiser from the University of Duisburg-Essen have identified small molecules that block specific components of the metabolic process like brake pads and prevent the downstream reactions. In their search for these molecules, they used a biological selection process involving intact plants, a technique borrowed from corporate drug research.
Kombrink, Kaiser and colleagues have identified a molecule that interferes with the effect of jasmonic acid. This plant hormone influences flower formation, root growth, defense against herbivores and infections, wound healing, ageing of plants, and much more.
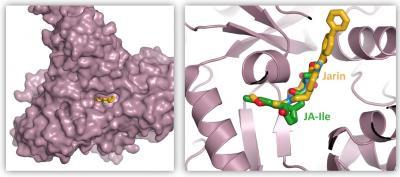
Jarin1 inhibits the enzyme JAR1 by displacing the natural substrate, jasmonoyl-isoleucine (JA-Ile), from its binding site. Both substances overlap, so that JAR1 can no longer fulfil its tasks. The left panel shows an overview of the entire enzyme; the right panel shows a view into the active centre. Credit: Corey S. Westfall, Washington University, St. Louis
Although many questions about plant metabolism can be answered through targeted gene mutations, the method has its limits. This is also demonstrated in the case of jasmonic acid and its derivatives. So far, only one signaling chain has been discovered, but this cannot explain the wide-ranging effect of this plant hormone. Therefore, other hitherto undiscovered signaling paths and action mechanisms must exist.
To find out more about them, Kombrink and Kaiser have adopted an approach that is similar to one used in medicine. Their strategy is based on the blocking of important metabolic pathways using low molecular weight compounds, which are easily assimilated by the plant. While in medical therapy such compounds are assimilated through the blood, in the plant they are introduced through the root.
The scientists embarked on their search with a screening of Arabidopsis thaliana and treating the plants with compounds in such a way that the desired selection could be identified by a conspicuous trait. Of the 1728 substances from a commercial compound library tested 16 emerged as inhibitors. This number was further reduced using more selective tests. In the end, only one substance turned out to be a specific inhibitor of the jasmonic acid signalling pathway and was given the name Jarin-1.
"In terms of its basic structure, the substance is a plant alkaloid, whose two amino groups can carry different side chains," Kombrink explains. "However, its effect is associated with a particular side chain in one of the positions. Other side chains impair the activity of the substance. We also deliberately synthesised it once again to be certain that we had understood its chemical structure correctly."
The scientists also looked for the target of the newly discovered inhibitor. The known signaling chain starts with the conjugation of the jasmonic acid with the amino acid isoleucine by an enzyme called JAR1. The resulting pair leads to the expression – following various detours – of the genes necessary for the relevant effect of the jasmonic acid. Kombrink and Kaiser were able to show that JAR1 is the target of the newly discovered inhibitor.
Due to the inhibition, the jasmonic acid conjugated with isoleucine does no longer accumulate in the cell. As a result genes are not expressed because the jasmonic acid–isoleucine pair no longer activates the genes' starting point.
The Jarin-1 inhibitor identified by Kombrink and Kaiser not only works in Arabidopsis but also in Cardamine hirsuta or hairy bittercress. "So we are obviously dealing with a broadly applicable molecule," comments Kombrink. Under the effect of the inhibitor, the plants show the same features as they do following the targeted mutation of genes from the jasmonic acid signalling pathway.
The scientists also investigated the exact location where the molecule takes effect. They succeeded in demonstrating that it binds to the active center of JAR1 and inhibits the natural substrate. "Our molecule is not a classical competitive inhibitor," says Kombrink. "But its effect can be explained, at least in parts, by displacement of the substrate from its binding site."
Small molecules are interesting new tools for plant research. Through their work, the researchers show how it is possible to search for them systematically and to identify their molecular mode of action.






Comments