We carry numerous bacteria on our skin, in our mouth, gut, and other tissues, and localized bacterial infections are common and mostly not harmful. Occasionally, however, a localized infection turns into dangerous systemic disease (sepsis), and scientists have new clues as to how that happens. A study published on March 20th in PLOS Pathogens shows that after intravenous injection of a million bacteria into a mouse, the resulting systemic disease is often started by only a single one of them.
Marco R. Oggioni, of the University of Leicester, UK, and colleagues studied the events that lead to systemic infection by Streptococcus pneumoniae (pneumococcus), a major human pathogen, in a mouse model. Consistent with earlier studies, they found that when streptococci are injected into immunologically naïve mice (whose immune systems had not been "educated" by previous encounter with the same pathogen) the vast majority of bacteria are destroyed rapidly by the host immune response. A dose of one million bacteria is needed to induce systemic disease in about half of the mice. This is in stark contrast to a much lower number of cells thought to make up the sepsis founder population, and the assumption is that there must be one or more "bottlenecks" in the development of sepsis.
To investigate these bottlenecks, the scientists injected mice with a mix of three different variants of S. pneumoniae. About half of the mice developed sepsis, and in almost all cases, the bacteria causing sepsis were derived from only one of the three variants. Using statistical analysis as well as direct DNA sequencing, the researchers could show that in most cases the bacterial population causing sepsis was started by a single pneumococcal cell. Their data also suggest that this "founder bacterium" had no obvious characteristics that gave it an advantage over the 999,999 others, but that random events determine which of the injected bacteria survives and multiplies to cause disease.
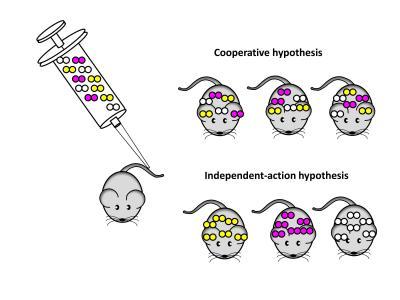
Rather than from a mixture of cooperating bacteria, sepsis seems to originate from a single bacterial cell.
(Photo Credit: Marco R. Oggioni)
When the researchers look closer at the mechanisms by which the immune system is able to clear most--but not always all--of the injected bacteria, they found that macrophages, a type of immune cell that can gobble up bacteria, and specifically macrophages in the spleen, are the main contributors to an efficient immune response. If bacteria survive that initial counter-attack by the host, a single founder bacterium multiplies and re-enters the bloodstream, where its descendants come under strong selective pressure that dynamically shapes the bacterial population, and thus causes the sepsis.
The researchers conclude "Although selective pressure generates diversity in bacterial populations during infection, invasive disease starts from a single founding cell which escapes initial immune clearance; a paradigm predicted to apply also to human systemic infections."






Comments